Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Single Amino Acid Substitution in the Vicinity of a Receptor-Binding Domain Changes Protein–Peptide Binding Affinity
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acsomega.7b00963 Galina Malovichko 1 , Xiangdong Zhu 1
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acsomega.7b00963 Galina Malovichko 1 , Xiangdong Zhu 1
Affiliation
|
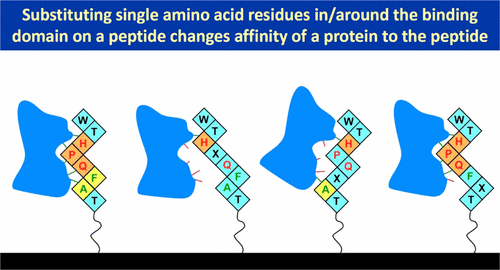
Using a microarray-based assay, we studied how the substitution of amino acids in the immediate vicinity of the receptor-binding domain on a peptide affects its binding to a protein. Replicates of 802 linear peptides consisting of the variants of WTHPQFAT and LQWHPQAGK, GKFPIPLGKQSG, and NGQFQVWIPGAQK, different by one amino acid, were synthesized on a glass slide with a maskless photolithography. Using a microarray-compatible label-free optical sensor, we measured the binding curves of streptavidin with the synthesized peptides and extracted the streptavidin–peptide affinity constants. We found that (a) the substitution of one residue in the HPQ motif reduces the affinity constant Ka from 108 M–1 by at least 3–4 orders of magnitude, with an exception of HPM; (b) substitution of the immediate flanking residue on the Gln side also causes the affinity to decrease by up to 3–4 orders of magnitude, depending on the substituting residue and the second-neighboring flanking residue; (c) substitution of the flanking residues on the His side has no significant effect on the affinity, possibly due to the strong binding of streptavidin to HPQF and HPQAG motifs. We also found that some of amino acid residues located close to the C-terminus (and the solid surface) improve the yield of peptide synthesis on a glass surface and can be exploited in the fabrication of peptide microarrays.
中文翻译:

受体结合域附近的单个氨基酸取代改变蛋白质-肽结合亲和力
使用基于微阵列的分析,我们研究了肽上受体结合结构域紧邻区域的氨基酸取代如何影响其与蛋白质的结合。在载玻片上用无掩模光刻法合成了由WT HPQ FAT和LQW HPQ AGK,GKFPIPLGKQSG和NGQFQVWIPGAQK的变体组成的802个线性肽的复制品,它们的差异为一个氨基酸。使用与微阵列兼容的无标记光学传感器,我们测量了抗生蛋白链菌素与合成肽的结合曲线,并提取了抗生蛋白链菌素-肽亲和常数。我们发现(a)HPQ基序中一个残基的取代降低了亲和常数K a从10 8 M–1至少3–4个数量级,HPM除外;(b)取代Gln侧的直接侧翼残基也会使亲和力降低多达3-4个数量级,具体取决于取代残基和第二个相邻的侧翼残基;(c)His侧的侧翼残基的取代对亲和力没有显着影响,这可能是由于链霉亲和素与HPQ F和HPQ AG基序的强结合。我们还发现,一些靠近C末端(和固体表面)的氨基酸残基提高了玻璃表面上肽合成的产率,可用于制造肽微阵列。
更新日期:2017-09-06
中文翻译:

受体结合域附近的单个氨基酸取代改变蛋白质-肽结合亲和力
使用基于微阵列的分析,我们研究了肽上受体结合结构域紧邻区域的氨基酸取代如何影响其与蛋白质的结合。在载玻片上用无掩模光刻法合成了由WT HPQ FAT和LQW HPQ AGK,GKFPIPLGKQSG和NGQFQVWIPGAQK的变体组成的802个线性肽的复制品,它们的差异为一个氨基酸。使用与微阵列兼容的无标记光学传感器,我们测量了抗生蛋白链菌素与合成肽的结合曲线,并提取了抗生蛋白链菌素-肽亲和常数。我们发现(a)HPQ基序中一个残基的取代降低了亲和常数K a从10 8 M–1至少3–4个数量级,HPM除外;(b)取代Gln侧的直接侧翼残基也会使亲和力降低多达3-4个数量级,具体取决于取代残基和第二个相邻的侧翼残基;(c)His侧的侧翼残基的取代对亲和力没有显着影响,这可能是由于链霉亲和素与HPQ F和HPQ AG基序的强结合。我们还发现,一些靠近C末端(和固体表面)的氨基酸残基提高了玻璃表面上肽合成的产率,可用于制造肽微阵列。



























 京公网安备 11010802027423号
京公网安备 11010802027423号