当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients – FINITE study
Journal of Hepatology ( IF 25.7 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.jhep.2017.07.012 Thomas Berg , Karl-Georg Simon , Stefan Mauss , Eckart Schott , Renate Heyne , Dietmar M. Klass , Christoph Eisenbach , Tania Mara Welzel , Reinhart Zachoval , Gisela Felten , Julian Schulze-zur-Wiesch , Markus Cornberg , Marjoleine L. Op den Brouw , Belinda Jump , Hans Reiser , Lothar Gallo , Tobias Warger , Jörg Petersen
Journal of Hepatology ( IF 25.7 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.jhep.2017.07.012 Thomas Berg , Karl-Georg Simon , Stefan Mauss , Eckart Schott , Renate Heyne , Dietmar M. Klass , Christoph Eisenbach , Tania Mara Welzel , Reinhart Zachoval , Gisela Felten , Julian Schulze-zur-Wiesch , Markus Cornberg , Marjoleine L. Op den Brouw , Belinda Jump , Hans Reiser , Lothar Gallo , Tobias Warger , Jörg Petersen
|
BACKGROUND & AIMS
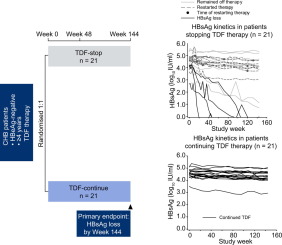
There is currently no virological cure for chronic hepatitis B but successful nucleos(t)ide analogue (NA) therapy can suppress hepatitis B virus (HBV) DNA replication and, in some cases, result in HBsAg loss. Stopping NA therapy often leads to viral relapse and therefore life-long therapy is usually required. This study investigated the potential to discontinue tenofovir disoproxil fumarate (TDF) therapy in HBeAg-negative patients. METHODS
Non-cirrhotic HBeAg-negative patients who had received TDF for ≥4years, with suppressed HBV DNA for ≥3.5years, were randomly assigned to either stop (n=21) or continue (n=21) TDF monotherapy. Standard laboratory tests including HBV DNA viral load, HBsAg and alanine aminotransferase (ALT) measurements, and adverse event reporting were carried out during treatment and post-treatment follow-up for 144weeks. RESULTS
Of the patients who stopped TDF therapy, 62% (n=13) remained off-therapy to Week 144. Median HBsAg change in this group was -0.59log10IU/ml (range -4.49 to 0.02log10IU/ml) vs. 0.21log10IU/ml in patients who continued TDF therapy. Four patients (19%) achieved HBsAg loss. Patients stopping therapy had initial fluctuations in viral load and ALT; however, at Week 144, 43% (n=9) had either achieved HBsAg loss or had HBV DNA <2,000IU/ml. There were no unexpected safety issues identified with stopping TDF therapy. CONCLUSIONS
This controlled study demonstrated the potential for HBsAg loss and/or sustained virological response in non-cirrhotic HBeAg-negative patients stopping long-term TDF therapy. Lay summary: Nucleos(t)ide analogue (NA) is usually a life-long therapy for HBV patients. This randomised controlled study investigated the discontinuation of tenofovir disoproxil fumarate (TDF) therapy in HBeAg-negative patients. Of the patients who stopped TDF therapy, 62% remained off-therapy to Week 144, of which 43% of patients had achieved either HBsAg loss or HBV DNA <2,000IU/ml. This offers a potential for long-term HBV-suppressed patients without cirrhosis to stop NA therapy under strict surveillance. Clinical trial number: NCT01320943.
中文翻译:

非肝硬化 HBeAg 阴性患者停用富马酸替诺福韦二吡呋酯后的长期反应 – FINITE 研究
背景和目的 目前慢性乙型肝炎没有病毒学治愈方法,但成功的核苷(酸)类似物(NA)治疗可以抑制乙型肝炎病毒(HBV)DNA 复制,在某些情况下,导致 HBsAg 消失。停止 NA 治疗通常会导致病毒复发,因此通常需要终生治疗。本研究调查了在 HBeAg 阴性患者中停用富马酸替诺福韦二吡呋酯 (TDF) 治疗的可能性。方法 接受 TDF ≥4 年且 HBV DNA 抑制≥3.5 年的非肝硬化 HBeAg 阴性患者被随机分配接受停止 (n=21) 或继续 (n=21) TDF 单药治疗。标准实验室检测,包括 HBV DNA 病毒载量、HBsAg 和丙氨酸转氨酶 (ALT) 测量值,治疗期间及治疗后随访144周进行不良事件报告。结果 在停止 TDF 治疗的患者中,62%(n=13)在第 144 周仍处于停药状态。该组的 HBsAg 变化中位数为 -0.59log10IU/ml(范围 -4.49 至 0.02log10IU/ml)与 0.21log10IU /ml 在继续 TDF 治疗的患者中。4 名患者 (19%) HBsAg 消失。停止治疗的患者最初的病毒载量和 ALT 波动;然而,在第 144 周时,43% (n=9) 或 HBsAg 消失或 HBV DNA <2,000IU/ml。没有发现停止 TDF 治疗的意外安全问题。结论 这项对照研究证明了在停止长期 TDF 治疗的非肝硬化 HBeAg 阴性患者中 HBsAg 消失和/或持续病毒学应答的潜力。奠定总结:核苷(酸)类似物 (NA) 通常是 HBV 患者的终生疗法。这项随机对照研究调查了 HBeAg 阴性患者停用富马酸替诺福韦二吡呋酯 (TDF) 治疗的情况。在停止 TDF 治疗的患者中,62% 的患者在第 144 周仍处于停药状态,其中 43% 的患者达到了 HBsAg 消失或 HBV DNA <2,000IU/ml。这为没有肝硬化的长期 HBV 抑制患者在严格监测下停止 NA 治疗提供了可能。临床试验编号:NCT01320943。其中 43% 的患者达到了 HBsAg 消失或 HBV DNA <2,000 IU/ml。这为没有肝硬化的长期 HBV 抑制患者在严格监测下停止 NA 治疗提供了可能。临床试验编号:NCT01320943。其中 43% 的患者达到了 HBsAg 消失或 HBV DNA <2,000 IU/ml。这为没有肝硬化的长期 HBV 抑制患者在严格监测下停止 NA 治疗提供了可能。临床试验编号:NCT01320943。
更新日期:2017-11-01
中文翻译:

非肝硬化 HBeAg 阴性患者停用富马酸替诺福韦二吡呋酯后的长期反应 – FINITE 研究
背景和目的 目前慢性乙型肝炎没有病毒学治愈方法,但成功的核苷(酸)类似物(NA)治疗可以抑制乙型肝炎病毒(HBV)DNA 复制,在某些情况下,导致 HBsAg 消失。停止 NA 治疗通常会导致病毒复发,因此通常需要终生治疗。本研究调查了在 HBeAg 阴性患者中停用富马酸替诺福韦二吡呋酯 (TDF) 治疗的可能性。方法 接受 TDF ≥4 年且 HBV DNA 抑制≥3.5 年的非肝硬化 HBeAg 阴性患者被随机分配接受停止 (n=21) 或继续 (n=21) TDF 单药治疗。标准实验室检测,包括 HBV DNA 病毒载量、HBsAg 和丙氨酸转氨酶 (ALT) 测量值,治疗期间及治疗后随访144周进行不良事件报告。结果 在停止 TDF 治疗的患者中,62%(n=13)在第 144 周仍处于停药状态。该组的 HBsAg 变化中位数为 -0.59log10IU/ml(范围 -4.49 至 0.02log10IU/ml)与 0.21log10IU /ml 在继续 TDF 治疗的患者中。4 名患者 (19%) HBsAg 消失。停止治疗的患者最初的病毒载量和 ALT 波动;然而,在第 144 周时,43% (n=9) 或 HBsAg 消失或 HBV DNA <2,000IU/ml。没有发现停止 TDF 治疗的意外安全问题。结论 这项对照研究证明了在停止长期 TDF 治疗的非肝硬化 HBeAg 阴性患者中 HBsAg 消失和/或持续病毒学应答的潜力。奠定总结:核苷(酸)类似物 (NA) 通常是 HBV 患者的终生疗法。这项随机对照研究调查了 HBeAg 阴性患者停用富马酸替诺福韦二吡呋酯 (TDF) 治疗的情况。在停止 TDF 治疗的患者中,62% 的患者在第 144 周仍处于停药状态,其中 43% 的患者达到了 HBsAg 消失或 HBV DNA <2,000IU/ml。这为没有肝硬化的长期 HBV 抑制患者在严格监测下停止 NA 治疗提供了可能。临床试验编号:NCT01320943。其中 43% 的患者达到了 HBsAg 消失或 HBV DNA <2,000 IU/ml。这为没有肝硬化的长期 HBV 抑制患者在严格监测下停止 NA 治疗提供了可能。临床试验编号:NCT01320943。其中 43% 的患者达到了 HBsAg 消失或 HBV DNA <2,000 IU/ml。这为没有肝硬化的长期 HBV 抑制患者在严格监测下停止 NA 治疗提供了可能。临床试验编号:NCT01320943。



























 京公网安备 11010802027423号
京公网安备 11010802027423号