Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2017-09-05 , DOI: 10.1016/j.apcata.2017.09.004 Denise Rein , Klaus Friedel Ortega , Claudia Weidenthaler , Eckhard Bill , Malte Behrens
|
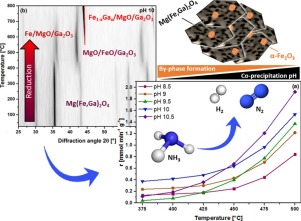
A series of mesoporous MgFe1.75Ga0.25O4 mixed spinel oxides obtained upon calcination of hydrotalcite-like precursors was investigated in the ammonia decomposition reaction at 1 atm after reduction in H2 atmosphere. The corresponding precursors were synthesized from metal salt solutions at five constant pH values in the range between 8.5 and 10.5 by co-precipitation in aqueous media to study the impact of pH variation on the catalyst’s structure and activity. N2 physisorption, thermogravimetric analysis, powder X-ray diffraction, Mössbauer spectroscopy, and temperature programmed techniques (H2-TPR and NH3-TPD) were applied to gather information about the textural, (micro-)structural, and adsorption properties of the samples. While phase purity in the precursor and oxide stages is only observed for pH = 10, undesired by-phases (MgFe2O4 and/or Fe3O4) are additionally formed during co-precipitation at the remaining pH values. This is partly related to an incomplete precipitation of Mg2+ cations in less alkaline environments. In situ XRD measurements during reduction revealed that Fe-Ga alloys are formed between 500 and 600 °C. The absence of by-phases avoids the formation of α-Fe, thus improving the structural and compositional homogeneity of the nitridated samples. This beneficial effect is reflected by the low activation energy (70 kJ/mol) and the enhanced low temperature activity (<450 °C) of the phase pure material in the catalytic decomposition of ammonia.



























 京公网安备 11010802027423号
京公网安备 11010802027423号