Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sorption Behavior of Bisphenol A and Triclosan by Graphene: Comparison with Activated Carbon
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1021/acsomega.7b00616 Fei Wang 1, 2, 3 , Xingwen Lu 1, 4 , Wenchao Peng 1, 5 , Yu Deng 1 , Tong Zhang 1 , Yibo Hu 1 , Xiao-yan Li 1
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1021/acsomega.7b00616 Fei Wang 1, 2, 3 , Xingwen Lu 1, 4 , Wenchao Peng 1, 5 , Yu Deng 1 , Tong Zhang 1 , Yibo Hu 1 , Xiao-yan Li 1
Affiliation
|
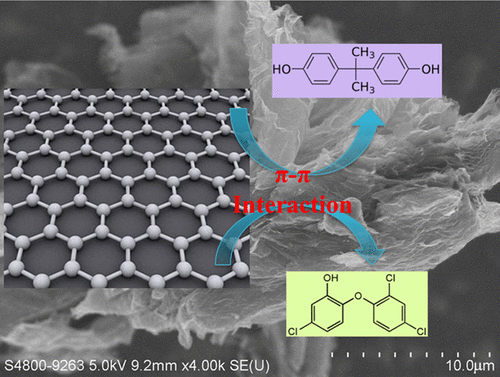
The sorption behavior of bisphenol A (BPA) and triclosan (TCS) on graphene was investigated and compared with that on activated carbon. The kinetic studies showed that BPA sorption on graphene or activated carbon reached equilibrium within 240 min, whereas TCS sorption on these two materials achieved equilibrium in 60 and 120 min. The maximum sorption capacity (qm) of BPA on graphene or activated carbon reached approximately 2.0 × 103 μg/g, which indicated that graphene was not superior to traditional activated carbon for BPA removal. By contrast, the strong partitioning ability of TCS on graphene suggested the potential use of graphene materials to remove TCS from wastewater. Although the pH change from 4.0 to 7.0 did not greatly affect BPA or TCS sorption, the sorption decreased dramatically when the pH was increased from 7.0 to 9.0. This phenomenon should be attributed to the establishment of electrostatic repulsion between anionic BPA (or TCS) molecules and the graphene (or activated carbon) surface under higher pH conditions. The increase of ion (NaCl and CaCl2) concentrations may lead to substantial increase of BPA sorption on graphene or activated carbon due to the salting-out effect. By contrast, ion concentrations had no significant effect on TCS sorption because of the dominant hydrophobic interaction.
中文翻译:

石墨烯对双酚A和三氯生的吸附行为:与活性炭的比较
研究了双酚A(BPA)和三氯生(TCS)在石墨烯上的吸附行为,并与活性炭进行了比较。动力学研究表明,BPA在石墨烯或活性炭上的吸附在240分钟内达到平衡,而TCS在这两种材料上的吸附在60和120分钟内达到平衡。BPA在石墨烯或活性炭上的最大吸附容量(q m)约为2.0×10 3μg/ g,这表明石墨烯在去除BPA方面并不优于传统的活性炭。相比之下,TCS在石墨烯上的强大分配能力表明,可以利用石墨烯材料从废水中去除TCS。尽管pH值从4.0更改为7.0不会极大地影响BPA或TCS的吸附,但当pH从7.0升高至9.0时,吸附量会急剧下降。此现象应归因于在较高pH条件下阴离子BPA(或TCS)分子与石墨烯(或活性炭)表面之间建立了静电排斥。离子(NaCl和CaCl 2的增加)的浓度可能会由于盐析效应而导致BPA在石墨烯或活性炭上的吸附量显着增加。相反,由于主要的疏水相互作用,离子浓度对TCS吸附没有显着影响。
更新日期:2017-09-05
中文翻译:

石墨烯对双酚A和三氯生的吸附行为:与活性炭的比较
研究了双酚A(BPA)和三氯生(TCS)在石墨烯上的吸附行为,并与活性炭进行了比较。动力学研究表明,BPA在石墨烯或活性炭上的吸附在240分钟内达到平衡,而TCS在这两种材料上的吸附在60和120分钟内达到平衡。BPA在石墨烯或活性炭上的最大吸附容量(q m)约为2.0×10 3μg/ g,这表明石墨烯在去除BPA方面并不优于传统的活性炭。相比之下,TCS在石墨烯上的强大分配能力表明,可以利用石墨烯材料从废水中去除TCS。尽管pH值从4.0更改为7.0不会极大地影响BPA或TCS的吸附,但当pH从7.0升高至9.0时,吸附量会急剧下降。此现象应归因于在较高pH条件下阴离子BPA(或TCS)分子与石墨烯(或活性炭)表面之间建立了静电排斥。离子(NaCl和CaCl 2的增加)的浓度可能会由于盐析效应而导致BPA在石墨烯或活性炭上的吸附量显着增加。相反,由于主要的疏水相互作用,离子浓度对TCS吸附没有显着影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号