JAMA Internal Medicine ( IF 39.0 ) Pub Date : 2017-09-01 , DOI: 10.1001/jamainternmed.2017.2719 Satyajit Reddy 1 , Vladimir Lakhter 2 , Chad J. Zack 3 , Huaqing Zhao 4 , Saurav Chatterjee 2 , Riyaz Bashir 2
|
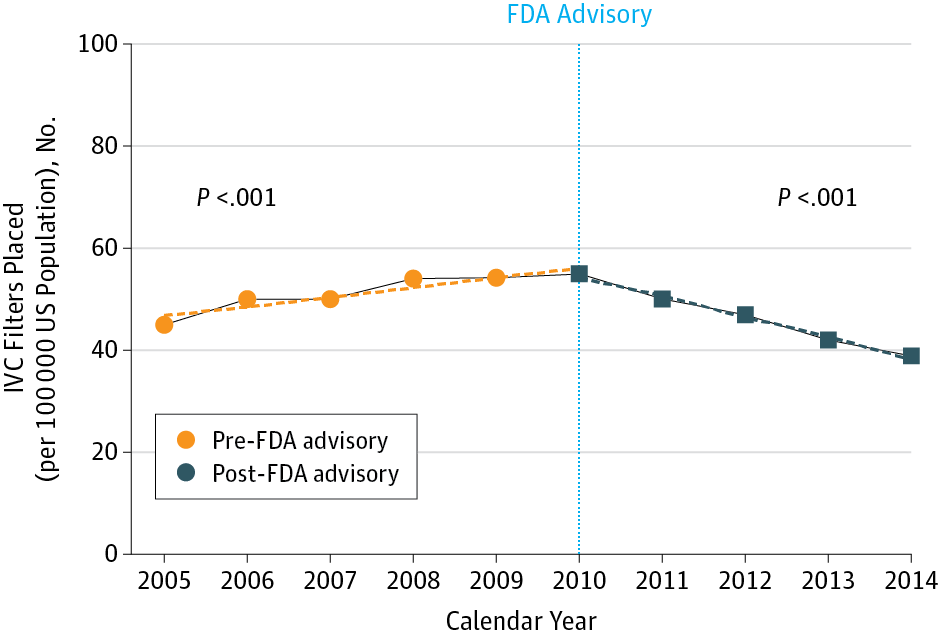
The effectiveness of inferior vena cava filter (IVCF) insertion in reducing venous thromboembolism (VTE)-associated morbidity and mortality is uncertain.1 Nevertheless, IVCF placement rates in the United States have been rapidly increasing and are 25-fold higher than in Europe.1 Prompted by the report by Nicholson et al2 in this journal of high prevalence of fracture and embolization, with potentially life-threatening sequelae of the Bard Recovery and Bard G2 IVC filters, the US Food and Drug Administration (FDA) issued a device safety warning on August 9, 2010, after reviewing 921 adverse events (ie, device migration, fracture, thrombosis) reported over a 5-year period.3 We sought to assess the nationwide utilization rates of IVCF placement in the United States and the impact of this FDA advisory. We also evaluated VTE-related hospitalization rates during the same period to determine whether any change in IVCF utilization could be accounted for by changes in VTE-related hospitalizations. Temple University waived the requirement for institutional review board approval.
中文翻译:

下腔静脉滤器放置的当代趋势与2010年美国食品药品监督管理局的联系
下腔静脉滤器(IVCF)插入在减少静脉血栓栓塞(VTE)相关的发病率和死亡率方面的有效性尚不确定。1然而,美国的IVCF安置率一直在迅速增长,比欧洲高25倍。1根据Nicholson等人的报告2在骨折和栓塞的高患病率报告中,加上Bard Recovery和Bard G2 IVC过滤器可能危及生命的后遗症,美国食品药品监督管理局(FDA)发布了设备安全性声明在审查了过去5年内报告的921次不良事件(例如器械迁移,骨折,血栓形成)后,于2010年8月9日发出警告。3我们试图评估美国IVCF放置的全国利用率以及该FDA咨询的影响。我们还评估了同期VTE相关的住院率,以确定IVTE相关住院的变化是否可以解释IVCF利用率的任何变化。天普大学放弃了机构审查委员会批准的要求。



























 京公网安备 11010802027423号
京公网安备 11010802027423号