Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2017-09-01 , DOI: 10.1016/j.bmc.2017.08.045 Gaopeng Song , Xiang Zhu , Junhua Li , Dekun Hu , Dongsheng Zhao , Yixian Liao , Juntong Lin , Lian-Hui Zhang , Zi-Ning Cui
|
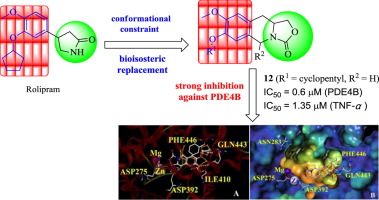
Improvement of subtype selectivity of an inhibitor’s binding activity using the conformational restriction approach has become an effective strategy in drug discovery. In this study, we applied this approach to PDE4 inhibitors and designed a series of novel oxazolidinone-fused 1,2,3,4-tetrahydroisoquinoline derivatives as conformationally restricted analogues of rolipram. The bioassay results demonstrated the oxazolidinone-fused tetrahydroisoquinoline derivatives exhibited moderate to good inhibitory activity against PDE4B and high selectivity for PDE4B/PDE4D. Among these derivatives, compound 12 showed both the strongest inhibition activity (IC50 = 0.60 μM) as well as good selectivity against PDE4B and good in vivo activity in animal models of asthma/COPD and sepsis induced by LPS. The primary SAR study showed that restricting the conformation of the catechol moiety in rolipram with the scaffold of oxazolidinone-fused tetrahydroisoquinoline could lead to an increase in selectivity for PDE4B over PDE4D, which was consistent with the observed docking simulation.
中文翻译:

构象受限的恶唑烷酮稠合的1,2,3,4-四氢异喹啉衍生物作为潜在的PDE4抑制剂的合理设计
使用构象限制方法改善抑制剂结合活性的亚型选择性已成为药物发现中的有效策略。在这项研究中,我们将这种方法应用于PDE4抑制剂,并设计了一系列新型恶唑烷酮稠合的1,2,3,4-四氢异喹啉衍生物作为咯利普兰的构象受限类似物。生物测定结果表明,恶唑烷酮融合的四氢异喹啉衍生物对PDE4B表现出中等至良好的抑制活性,对PDE4B / PDE4D的选择性高。在这些衍生物中,化合物12表现出最强的抑制活性(IC 50 = 0.60μM)以及对PDE4B的良好选择性和良好的体内活性LPS诱发的哮喘/ COPD和败血症动物模型中的活性降低。SAR的主要研究表明,用恶唑烷酮融合的四氢异喹啉骨架限制咯利普兰中邻苯二酚部分的构象可能导致PDE4B的选择性比PDE4D有所提高,这与观察到的对接模拟一致。


























 京公网安备 11010802027423号
京公网安备 11010802027423号