Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2017-08-31 , DOI: 10.1016/j.bmc.2017.08.017 Matthew P. Sarnowski , Kyle P. Pedretty , Nicole Giddings , H. Lee Woodcock , Juan R. Del Valle
|
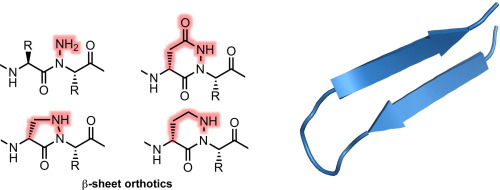
The stabilization of β-sheet secondary structure through peptide backbone modification represents an attractive approach to protein mimicry. Here, we present strategies toward stable β-hairpin folds based on peptide strand N-amination. Novel pyrazolidinone and tetrahydropyridazinone dipeptide constraints were introduced via on-resin Mitsunobu cyclization between α-hydrazino acid residues and a serine or homoserine side chain. Acyclic and cyclic N-amino peptide building blocks were then evaluated for their effect on β-hairpin stability in water using a GB1-derived model system. Our results demonstrate the strong β-sheet stabilizing effect of the peptide N-amino substituent, and provide useful insights into the impact of covalent dipeptide constraint on β-sheet folding.
中文翻译:

约束N-氨基肽的合成及其β-折叠倾向
通过肽主链修饰来稳定β-折叠二级结构代表了一种模仿蛋白质的诱人方法。在这里,我们提出了基于肽链N-氨基化的稳定β-发夹折叠的策略。通过在α-肼基酸残基与丝氨酸或高丝氨酸侧链之间的Mitsunobu树脂上的环化作用,引入了新的吡唑烷酮和四氢哒嗪酮二肽约束条件。然后使用GB1衍生的模型系统评估无环和环状N-氨基肽结构单元对水中β-发夹稳定性的影响。我们的结果证明了肽N具有很强的β折叠稳定作用-氨基取代基,并为共价二肽约束对β-折叠折叠的影响提供有用的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号