Tetrahedron ( IF 2.1 ) Pub Date : 2017-09-04 , DOI: 10.1016/j.tet.2017.08.056 Kazuaki Kuwata , Kengo Hanaya , Shuhei Higashibayashi , Takeshi Sugai , Mitsuru Shoji
|
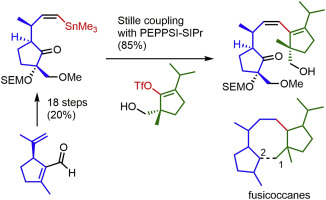
1-Hydroxy-14-isopropyl-3β-methoxymethyl-7β,11β-dimethyl-3α-[((2-trimethylsilyl)ethoxy)methoxy]-1,2-secofusicocca-8,10(14)-dien-2-one, a highly functionalized 1,2-seco fusicoccane diterpene skeleton related to cotylenin A was synthesized in a convergent manner. The A ring segment, i. e., (1′R,2S, 2′E,5S)-2-methoxymethyl-5-[1′-methyl-3’-(trimethylstannyl)prop-2-enyl]-2-[((2″-trimethylsilyl)ethoxy)methoxy]cyclopentanone, was synthesized in 20.1% yield over 18 steps from known (S)-5-isopropenyl-2-methylcyclopent-1-enecarbaldehyde. This was coupled with the C ring segment, i. e., (R)-5-hydroxymethyl-2-isopropyl-5-methylcyclopent-1-en-1-yl trifluoromethylsulfonate, which was prepared according to our previous report. The Stille coupling reaction between alkenylstannane and sterically hindered triflate proceeded successfully in the presence of PEPPSI-SIPr (85%), and the total yield of the target molecule was 17.1% over the longest linear sequences (19 steps) from (S)-5-isopropenyl-2-methylcyclopent-1-enecarbaldehyde.
中文翻译:

通过辅酶A的高度官能化的A和C环段之间的Stille偶联反应合成1,2-seco富二烷烷二萜骨架
1-羟基-14-异丙基-3β-甲氧基甲基-7β,11β-二甲基-3α-[(((2-三甲基甲硅烷基)乙氧基)甲氧基] -1,2-仲水杨酸8,10(14)-二-2- ,以收敛的方式合成了与辅肾素A有关的高度官能化的1,2-seco呋喃二烷二萜骨架。A环段,即。e。,(1'R,2 S,2'E,5 S)-2-甲氧基甲基-5- [1'-甲基-3'-(三甲基锡烷基)丙-2-烯基] -2-[((从已知的(S)-5-异丙烯基-2-甲基环戊-1-烯甲醛经18步合成了“-三甲基甲硅烷基)乙氧基)甲氧基]环戊酮。这与C环段,即。e。,(R)-5-羟甲基-2-异丙基-5-甲基环戊-1-烯-1-基三氟甲基磺酸盐,它是根据我们以前的报告制备的。在PEPPSI-SIPr(85%)的存在下,烯基锡烷与空间受阻的三氟甲磺酸酯之间的Stille偶联反应成功进行,在(S)-5的最长线性序列(19个步骤)中,目标分子的总产率为17.1%-异丙烯基-2-甲基环戊-1-烯甲醛。


























 京公网安备 11010802027423号
京公网安备 11010802027423号