Tetrahedron ( IF 2.1 ) Pub Date : 2017-08-31 , DOI: 10.1016/j.tet.2017.08.049 Ruo-Heng Su , Xiang-Feng Ding , Shu-Xiao Wu , Jian-Hong Zhao , Wei-Ping Deng
|
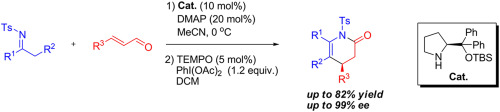
An amine-catalyzed asymmetric formal aza [3 + 3] cycloaddition of α,β-unsaturated aldehydes with N-Ts ketimines derived from both acyclic and cyclic ketones was developed, which was followed by an oxidation to afford chiral piperidine derivatives in good yields and excellent enantioselectivities (up to 99% ee). In addition, the corresponding cycloadduct piperidin-2-ols can be easily transformed to indolyl substituted chiral piperidine derivatives in good yields and excellent diastereoselectivities (>20:1) via Friedel-Crafts alkylation of indole in the presence of BF3·Et2O.
中文翻译:

仲胺催化的不对称形式氮杂[3 + 3]环加成反应,构建对映体富集的哌啶衍生物
开发了一种胺催化的α,β-不饱和醛与不饱和酮和环状酮衍生的N -Ts酮亚胺的不对称正氮杂[3 + 3]环加成反应,随后进行氧化反应以提供高收率的手性哌啶衍生物优异的对映选择性(高达99%ee)。此外,在BF 3 ·Et 2 O存在下,通过吲哚的Friedel-Crafts烷基化,可以轻松地以良好的收率和优异的非对映选择性(> 20:1)将相应的环加合物哌啶-2-醇转化为吲哚基取代的手性哌啶衍生物。。


























 京公网安备 11010802027423号
京公网安备 11010802027423号