当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cationic Scandium Anisyl Species in Styrene Polymerization Using Anisole and N,N-Dimethyl-o-toluidine as Chain-Transfer Agents
Organometallics ( IF 2.8 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.organomet.7b00526 Atsushi Yamamoto 1 , Masayoshi Nishiura 1, 2 , Yang Yang 2 , Zhaomin Hou 1, 2
Organometallics ( IF 2.8 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.organomet.7b00526 Atsushi Yamamoto 1 , Masayoshi Nishiura 1, 2 , Yang Yang 2 , Zhaomin Hou 1, 2
Affiliation
|
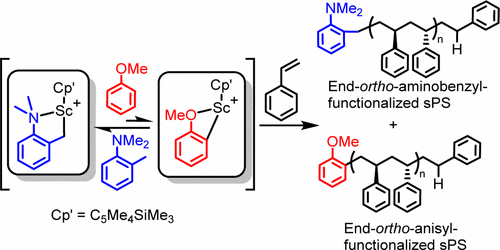
This work aimed to clarify the involvement of a cationic scandium anisyl species in the scandium-catalyzed chain-transfer polymerization of styrene using anisole as a chain-transfer agent (CTA) as well as its behavior in the presence of N,N-dimethyl-o-toluidine. The reaction of anisole with the catalyst precursor [(C5Me4SiMe3)Sc(CH2C6H4NMe2-o)][B(C6F5)4] (2) generated by the reaction of (C5Me4SiMe3)Sc(CH2C6H4NMe2-o)2 (1) with [Ph3C][B(C6F5)4] gave the structurally characterizable anisole-coordinated ion-pair complex [(C5Me4SiMe3)Sc(CH2C6H4NMe2-o)(C6H5OMe)][B(C6F5)4] (3). The aminobenzyl unit in 3 remained intact even in the presence of an excess amount of anisole. The formation of an anisyl species from 3 was not observed by 1H NMR. However, the polymerization of styrene by 3 at either room temperature or 70 °C yielded the anisyl-end-functionalized syndiotactic polystyrene (sPS) as a major product in addition to the dimethylaminobenzyl-end-capped sPS. These results suggest that an equilibrium between the aminobenzyl species 3 and a scandium anisyl species should exist, although the latter was not detected by 1H NMR. The reaction of the half-sandwich scandium bis(anisyl) complex (C5Me4SiMe3)Sc(C6H4OMe-o)2 (4) with 1 equiv of [Ph3C][B(C6F5)4] in THF afforded the THF-coordinated cationic scandium monoanisyl complex [(C5Me4SiMe3)Sc(C6H4OMe-o)(thf)2][B(C6F5)4] (5), which upon reaction with HMPA gave the structurally characterizable HMPA-coordinated analogue [(C5Me4SiMe3)Sc(C6H4OMe-o)(hmpa)2][B(C6F5)4] (6). The 4/[Ph3C][B(C6F5)4] combination in toluene showed high activity for styrene polymerization, selectively yielding the anisyl-end-functionalized sPS. In addition to anisole, N,N-dimethyl-o-toluidine was also found to serve as an efficient CTA for the polymerization of styrene by 1/[Ph3C][B(C6F5)4], which selectively afforded the aminobenzyl-functionalized sPS.
中文翻译:

苯甲醚和N,N-二甲基-邻甲苯胺为链转移剂的苯乙烯聚合中的阳离子Scan茴香基物种
这项工作旨在阐明阳离子scan茴香基物种在使用苯甲醚作为链转移剂(CTA)的苯乙烯的of催化链转移聚合中的参与及其在N,N-二甲基-邻甲苯胺。苯甲醚与催化剂前体反应〔(C 5我4森达3)钪(CH 2 ç 6 ħ 4 NME 2 - ö)] [B(C 6 ˚F 5)4 ](2)由反应生成的( C 5 Me 4 SiMe3)Sc(CH 2 C 6 H 4 NMe 2 - o)2(1)与[Ph 3 C] [B(C 6 F 5)4 ]给出结构可表征的苯甲醚配位离子对络合物[(C 5 Me 4 SiMe 3)Sc(CH 2 C 6 H 4 NMe 2 - o)(C 6 H 5 OMe)] [B(C 6 F 5)4 ](3)。即使在过量的茴香醚存在下,3中的氨基苄基单元仍保持完整。通过1 H NMR未观察到由3形成茴香基物质。然而,在室温或70°C下,苯乙烯在3的温度下聚合,除了以二甲基氨基苄基封端的sPS外,还产生了以茴香基端官能化的间规聚苯乙烯(sPS)为主要产物。这些结果表明,尽管没有通过1 H NMR检测到后者,但是应该在氨基苄基物种3和a茴香基物种之间存在平衡。半三明治scan双(茴香基)络合物(C 5 Me 4 SiMe3)具有1当量[ THF ]的[Ph 3 C] [B(C 6 F 5)4 ]的Sc(C 6 H 4 OMe- o)2(4)提供了THF配位的阳离子scan单茴香基络合物[(C 5 Me 4 SiMe 3)Sc(C 6 H 4 OMe- o)(thf)2 ] [B(C 6 F 5)4 ](5),与HMPA反应后得到结构上可表征的HMPA配位类似物[[C 5我4森达3)钪(C 6 H ^ 4 OMe- ö)(HMPA)2 ] [B(C 6 ˚F 5)4 ](6)。甲苯中的4 / [Ph 3 C] [B(C 6 F 5)4 ]组合物对苯乙烯聚合显示出高活性,有选择地生成经茴香基末端官能化的sPS。除苯甲醚外,还发现N,N-二甲基邻甲苯胺可作为有效的CTA,用于通过1 / [Ph 3 C] [B(C)聚合苯乙烯。6 F 5)4 ],其选择性地提供了氨基苄基官能化的sPS。
更新日期:2017-08-31
中文翻译:

苯甲醚和N,N-二甲基-邻甲苯胺为链转移剂的苯乙烯聚合中的阳离子Scan茴香基物种
这项工作旨在阐明阳离子scan茴香基物种在使用苯甲醚作为链转移剂(CTA)的苯乙烯的of催化链转移聚合中的参与及其在N,N-二甲基-邻甲苯胺。苯甲醚与催化剂前体反应〔(C 5我4森达3)钪(CH 2 ç 6 ħ 4 NME 2 - ö)] [B(C 6 ˚F 5)4 ](2)由反应生成的( C 5 Me 4 SiMe3)Sc(CH 2 C 6 H 4 NMe 2 - o)2(1)与[Ph 3 C] [B(C 6 F 5)4 ]给出结构可表征的苯甲醚配位离子对络合物[(C 5 Me 4 SiMe 3)Sc(CH 2 C 6 H 4 NMe 2 - o)(C 6 H 5 OMe)] [B(C 6 F 5)4 ](3)。即使在过量的茴香醚存在下,3中的氨基苄基单元仍保持完整。通过1 H NMR未观察到由3形成茴香基物质。然而,在室温或70°C下,苯乙烯在3的温度下聚合,除了以二甲基氨基苄基封端的sPS外,还产生了以茴香基端官能化的间规聚苯乙烯(sPS)为主要产物。这些结果表明,尽管没有通过1 H NMR检测到后者,但是应该在氨基苄基物种3和a茴香基物种之间存在平衡。半三明治scan双(茴香基)络合物(C 5 Me 4 SiMe3)具有1当量[ THF ]的[Ph 3 C] [B(C 6 F 5)4 ]的Sc(C 6 H 4 OMe- o)2(4)提供了THF配位的阳离子scan单茴香基络合物[(C 5 Me 4 SiMe 3)Sc(C 6 H 4 OMe- o)(thf)2 ] [B(C 6 F 5)4 ](5),与HMPA反应后得到结构上可表征的HMPA配位类似物[[C 5我4森达3)钪(C 6 H ^ 4 OMe- ö)(HMPA)2 ] [B(C 6 ˚F 5)4 ](6)。甲苯中的4 / [Ph 3 C] [B(C 6 F 5)4 ]组合物对苯乙烯聚合显示出高活性,有选择地生成经茴香基末端官能化的sPS。除苯甲醚外,还发现N,N-二甲基邻甲苯胺可作为有效的CTA,用于通过1 / [Ph 3 C] [B(C)聚合苯乙烯。6 F 5)4 ],其选择性地提供了氨基苄基官能化的sPS。



























 京公网安备 11010802027423号
京公网安备 11010802027423号