当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Assembling Glycan-Charged Dolichol Phosphates: Chemoenzymatic Synthesis of a Haloferax volcanii N-Glycosylation Pathway Intermediate
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00436 Yifat Elharar 1 , Ananda Rao Podilapu 2 , Ziqiang Guan 3 , Suvarn S. Kulkarni 2 , Jerry Eichler 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00436 Yifat Elharar 1 , Ananda Rao Podilapu 2 , Ziqiang Guan 3 , Suvarn S. Kulkarni 2 , Jerry Eichler 1
Affiliation
|
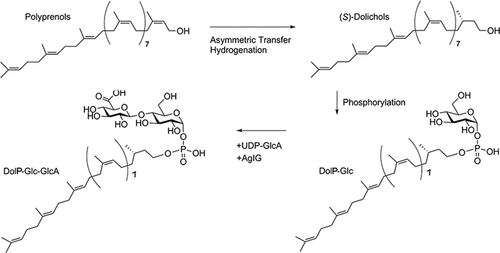
N-glycosylation, the covalent attachment of glycans to select protein target Asn residues, is a post-translational modification performed by all three domains of life. In the halophilic archaea Haloferax volcanii, in which understanding of this universal protein-processing event is relatively well-advanced, genes encoding the components of the archaeal glycosylation (Agl) pathway responsible for the assembly and attachment of an N-linked pentasaccharide have been identified. As elsewhere, the N-linked glycan is assembled on phosphodolichol carriers before transfer to target Asn residues. However, as little is presently known of the Hfx. volcanii Agl pathway at the protein level, the seemingly unique ability of Archaea to use dolichol phosphate (DolP) as the glycan lipid carrier, rather than dolichol pyrophosphate used by eukaryotes, remains poorly understood. With this in mind, a chemoenzymatic approach was taken to biochemically study AglG, one of the five glycosyltransferases of the pathway. Accordingly, a novel regio- and stereoselective reduction of naturally isolated polyprenol gave facile access to S-dolichol via asymmetric transfer hydrogenation under very mild conditions. This compound was used to generate glucose-charged DolP, a precursor of the N-linked pentasaccharide, as well as DolP–glucose–glucuronic acid and DolP–glucuronic acid. AglG, purified from Hfx. volcanii membranes in hypersaline conditions, like those encountered in situ, was subsequently combined with uridine diphosphate (UDP)–glucuronic acid and DolP–glucose to yield DolP–glucose–glucuronic acid. The in vitro system for the study of AglG activity developed here represents the first such tool for studying halophilic glycosyltransferases and will allow for a detailed understanding of archaeal N-glycosylation.
中文翻译:

组装聚糖带电的多羟基磷酸酯:嗜盐菌(Haloferax volcanii) N-糖基化途径中间体的化学酶法合成
N-糖基化,即聚糖选择蛋白质目标Asn残基的聚糖共价连接,是生命的所有三个结构域进行的翻译后修饰。在嗜盐古生菌Haloferax volcanii中,对这种普遍的蛋白质加工事件的理解相对较先进,已经鉴定出编码古细菌糖基化(Agl)途径中负责N-连接五糖组装和附着的成分的基因。 。与其他地方一样,在转移至目标Asn残基之前,将N-连接的聚糖组装在磷酸二氢乙醇载体上。但是,目前对Hfx知之甚少。火山在蛋白质水平上的Ag1途径,古细菌似乎使用磷酸二氢乙醇酯(DolP)作为聚糖脂质载体,而不是真核生物所用的焦磷酸二氢大麻酚的看似独特能力,仍然知之甚少。考虑到这一点,采用化学酶学方法对该途径的五个糖基转移酶之一AglG进行了生物化学研究。因此,在非常温和的条件下,通过自然分离的聚戊二烯的新的区域和立体选择性还原,可以通过不对称转移氢化而容易地获得S-二羟戊醇。该化合物用于生成带有葡萄糖的DolP(N连接的五糖的前体)以及DolP-葡萄糖-葡萄糖醛酸和DolP-葡萄糖醛酸。从Hfx纯化的AglG 。火山随后将高盐条件下的膜(如原位遇到的膜)与尿苷二磷酸(UDP)-葡萄糖醛酸和DolP-葡萄糖结合,生成DolP-葡萄糖-葡萄糖醛酸。这里开发的用于研究Ag1G活性的体外系统代表了第一个用于研究嗜盐糖基转移酶的工具,并且将允许对古细菌N-糖基化的详细了解。
更新日期:2017-08-30
中文翻译:

组装聚糖带电的多羟基磷酸酯:嗜盐菌(Haloferax volcanii) N-糖基化途径中间体的化学酶法合成
N-糖基化,即聚糖选择蛋白质目标Asn残基的聚糖共价连接,是生命的所有三个结构域进行的翻译后修饰。在嗜盐古生菌Haloferax volcanii中,对这种普遍的蛋白质加工事件的理解相对较先进,已经鉴定出编码古细菌糖基化(Agl)途径中负责N-连接五糖组装和附着的成分的基因。 。与其他地方一样,在转移至目标Asn残基之前,将N-连接的聚糖组装在磷酸二氢乙醇载体上。但是,目前对Hfx知之甚少。火山在蛋白质水平上的Ag1途径,古细菌似乎使用磷酸二氢乙醇酯(DolP)作为聚糖脂质载体,而不是真核生物所用的焦磷酸二氢大麻酚的看似独特能力,仍然知之甚少。考虑到这一点,采用化学酶学方法对该途径的五个糖基转移酶之一AglG进行了生物化学研究。因此,在非常温和的条件下,通过自然分离的聚戊二烯的新的区域和立体选择性还原,可以通过不对称转移氢化而容易地获得S-二羟戊醇。该化合物用于生成带有葡萄糖的DolP(N连接的五糖的前体)以及DolP-葡萄糖-葡萄糖醛酸和DolP-葡萄糖醛酸。从Hfx纯化的AglG 。火山随后将高盐条件下的膜(如原位遇到的膜)与尿苷二磷酸(UDP)-葡萄糖醛酸和DolP-葡萄糖结合,生成DolP-葡萄糖-葡萄糖醛酸。这里开发的用于研究Ag1G活性的体外系统代表了第一个用于研究嗜盐糖基转移酶的工具,并且将允许对古细菌N-糖基化的详细了解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号