当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inter- and Intramolecular Bonding in 1,3,5-Triamino-2,4,6-trinitrobenzene: An Experimental and Theoretical Quantum Theory of Atoms in Molecules (QTAIM) Analysis
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.cgd.7b00674 Zhijie Chua 1 , Christopher G. Gianopoulos 1 , Bartosz Zarychta 1, 2 , Elizabeth A. Zhurova 1 , Vladimir V. Zhurov 1 , A. Alan Pinkerton 1
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2017-08-30 00:00:00 , DOI: 10.1021/acs.cgd.7b00674 Zhijie Chua 1 , Christopher G. Gianopoulos 1 , Bartosz Zarychta 1, 2 , Elizabeth A. Zhurova 1 , Vladimir V. Zhurov 1 , A. Alan Pinkerton 1
Affiliation
|
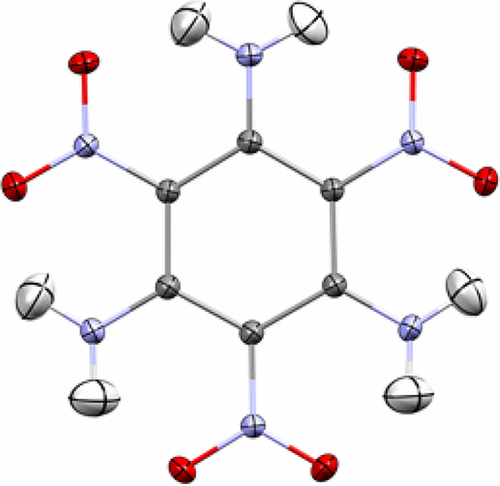
Chemical bonding in the triclinic phase of 1,3,5-triamino-2,4,6-trinitrobenzene (TATB) has been analyzed based on the experimental electron density derived from X-ray diffraction data obtained at 20 K. The results have been compared with those from solid state theoretical calculations. The total electron density has been analyzed in terms of the Quantum Theory of Atoms in Molecules (QTAIM). Features of the covalent bonds demonstrate the presence of multiple bonds of various order. Strong intramolecular hydrogen bonds and weaker intermolecular bonds within the layer structure are characterized by the properties of their (3, −1) critical points. Weaker interactions, predominantly O···O, between the layers have additionally been characterized. Integrated atomic charges are also reported. The importance of correcting the primary X-ray data for λ/2 contamination is discussed.
中文翻译:

1,3,5-三氨基-2,4,6-三硝基苯中的分子间和分子内键合:分子中原子的实验和理论量子理论(QTAIM)分析
根据在20 K下获得的X射线衍射数据得出的实验电子密度,对1,3,5-三氨基-2,4,6-三硝基苯(TATB)的三斜晶相中的化学键进行了分析。与固态理论计算得出的结果进行了比较。已根据分子中的原子量子理论(QTAIM)分析了总电子密度。共价键的特征表明存在多个不同顺序的键。层结构内的强分子内氢键和弱分子间键的特征在于其(3,-1)临界点的性质。层之间的相互作用较弱,主要是O···O。还报告了集成原子电荷。
更新日期:2017-08-30
中文翻译:

1,3,5-三氨基-2,4,6-三硝基苯中的分子间和分子内键合:分子中原子的实验和理论量子理论(QTAIM)分析
根据在20 K下获得的X射线衍射数据得出的实验电子密度,对1,3,5-三氨基-2,4,6-三硝基苯(TATB)的三斜晶相中的化学键进行了分析。与固态理论计算得出的结果进行了比较。已根据分子中的原子量子理论(QTAIM)分析了总电子密度。共价键的特征表明存在多个不同顺序的键。层结构内的强分子内氢键和弱分子间键的特征在于其(3,-1)临界点的性质。层之间的相互作用较弱,主要是O···O。还报告了集成原子电荷。


























 京公网安备 11010802027423号
京公网安备 11010802027423号