当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Increasing Oxide Reducibility: The Role of Metal/Oxide Interfaces in the Formation of Oxygen Vacancies
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1021/acscatal.7b01913 Antonio Ruiz Puigdollers 1 , Philomena Schlexer 1 , Sergio Tosoni 1 , Gianfranco Pacchioni 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1021/acscatal.7b01913 Antonio Ruiz Puigdollers 1 , Philomena Schlexer 1 , Sergio Tosoni 1 , Gianfranco Pacchioni 1
Affiliation
|
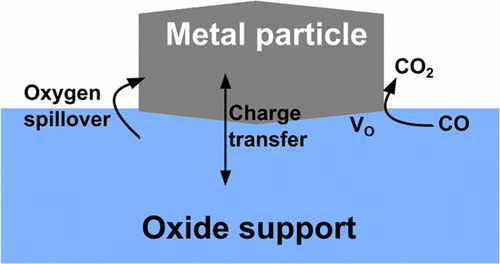
Reducibility is an essential characteristic of oxide catalysts in oxidation reactions following the Mars–van Krevelen mechanism. A typical descriptor of the reducibility of an oxide is the cost of formation of an oxygen vacancy, which measures the tendency of the oxide to lose oxygen or to donate it to an adsorbed species with consequent change in the surface composition, from MnOm to MnOm–x. The oxide reducibility, however, can be modified in various ways: for instance, by doping and/or nanostructuring. In this review we consider an additional aspect, related to the formation of a metal/oxide interface. This can be realized when small metal nanoparticles are deposited on the surface of an oxide support or when a nanostructured oxide, either a nanoparticle or a thin film, is grown on a metal. In the past decade, both theory and experiment indicate a particularly high reactivity of the oxygen atoms at the boundary region between a metal and an oxide. Oxygen atoms can be removed from interface sites at much lower cost than in other regions of the surface. This can alter completely the reactivity of a solid catalyst. In this respect, reducibility of the bulk material may differ completely from that of the metal/oxide surface. The atomistic study of CO oxidation and water-gas shift reactions are used as examples to provide compelling evidence that the oxidation occurs at specific interface sites, the actual active sites in the complex catalyst. Combining oxide nanostructuring with metal/oxide interfaces opens promising perspectives to turn hardly reducible oxides into reactive materials in oxidation reactions based on the Mars–van Krevelen mechanism.
中文翻译:

提高氧化物还原性:金属/氧化物界面在氧空位形成中的作用
可还原性是遵循Mars–van Krevelen机理的氧化反应中氧化物催化剂的基本特征。氧化物可还原性的典型描述是形成氧空位的成本,该空位衡量了氧化物失去氧气或将其捐赠给被吸附物质的趋势,因此表面组成发生了变化,由M n O m至M n O m – x。然而,可以以各种方式来改变氧化物的可还原性:例如,通过掺杂和/或纳米结构化。在这篇综述中,我们考虑了与金属/氧化物界面形成有关的另一个方面。当小的金属纳米颗粒沉积在氧化物载体的表面上时,或者当纳米结构的氧化物(纳米颗粒或薄膜)生长在金属上时,就可以实现这一点。在过去的十年中,理论和实验都表明,氧原子在金属和氧化物之间的边界区域的反应性特别高。可以以比表面的其他区域低得多的成本从界面位点除去氧原子。这可以完全改变固体催化剂的反应性。在这方面,块状材料的可还原性可与金属/氧化物表面的可还原性完全不同。以CO氧化和水煤气变换反应的原子研究为例,提供了令人信服的证据,证明氧化发生在特定的界面部位,即复杂催化剂中的实际活性部位。将氧化物纳米结构与金属/氧化物界面结合在一起,为基于Mars–van Krevelen机理在氧化反应中将几乎不可还原的氧化物转变为反应性材料提供了广阔的前景。
更新日期:2017-08-30
中文翻译:

提高氧化物还原性:金属/氧化物界面在氧空位形成中的作用
可还原性是遵循Mars–van Krevelen机理的氧化反应中氧化物催化剂的基本特征。氧化物可还原性的典型描述是形成氧空位的成本,该空位衡量了氧化物失去氧气或将其捐赠给被吸附物质的趋势,因此表面组成发生了变化,由M n O m至M n O m – x。然而,可以以各种方式来改变氧化物的可还原性:例如,通过掺杂和/或纳米结构化。在这篇综述中,我们考虑了与金属/氧化物界面形成有关的另一个方面。当小的金属纳米颗粒沉积在氧化物载体的表面上时,或者当纳米结构的氧化物(纳米颗粒或薄膜)生长在金属上时,就可以实现这一点。在过去的十年中,理论和实验都表明,氧原子在金属和氧化物之间的边界区域的反应性特别高。可以以比表面的其他区域低得多的成本从界面位点除去氧原子。这可以完全改变固体催化剂的反应性。在这方面,块状材料的可还原性可与金属/氧化物表面的可还原性完全不同。以CO氧化和水煤气变换反应的原子研究为例,提供了令人信服的证据,证明氧化发生在特定的界面部位,即复杂催化剂中的实际活性部位。将氧化物纳米结构与金属/氧化物界面结合在一起,为基于Mars–van Krevelen机理在氧化反应中将几乎不可还原的氧化物转变为反应性材料提供了广阔的前景。


























 京公网安备 11010802027423号
京公网安备 11010802027423号