当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Engineering Antibody Reactivity for Efficient Derivatization to Generate NaV1.7 Inhibitory GpTx-1 Peptide–Antibody Conjugates
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1021/acschembio.7b00542 Kaustav Biswas 1, 2 , Thomas E. Nixey 1, 2 , Justin K. Murray 1, 2 , James R. Falsey 1, 2 , Li Yin 1, 2 , Hantao Liu 1, 2 , Jacinthe Gingras 1, 2 , Brian E. Hall 1, 2 , Brad Herberich 1, 2 , Jerry Ryan Holder 1, 2 , Hongyan Li 1, 2 , Joseph Ligutti 1, 2 , Min-Hwa Jasmine Lin 1, 2 , Dong Liu 1, 2 , Brian D. Soriano 1, 2 , Marcus Soto 1, 2 , Linh Tran 1, 2 , Christopher M. Tegley 1, 2 , Anrou Zou 1, 2 , Kannan Gunasekaran 1, 2 , Bryan D. Moyer 1, 2 , Liz Doherty 1, 2 , Les P. Miranda 1, 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1021/acschembio.7b00542 Kaustav Biswas 1, 2 , Thomas E. Nixey 1, 2 , Justin K. Murray 1, 2 , James R. Falsey 1, 2 , Li Yin 1, 2 , Hantao Liu 1, 2 , Jacinthe Gingras 1, 2 , Brian E. Hall 1, 2 , Brad Herberich 1, 2 , Jerry Ryan Holder 1, 2 , Hongyan Li 1, 2 , Joseph Ligutti 1, 2 , Min-Hwa Jasmine Lin 1, 2 , Dong Liu 1, 2 , Brian D. Soriano 1, 2 , Marcus Soto 1, 2 , Linh Tran 1, 2 , Christopher M. Tegley 1, 2 , Anrou Zou 1, 2 , Kannan Gunasekaran 1, 2 , Bryan D. Moyer 1, 2 , Liz Doherty 1, 2 , Les P. Miranda 1, 2
Affiliation
|
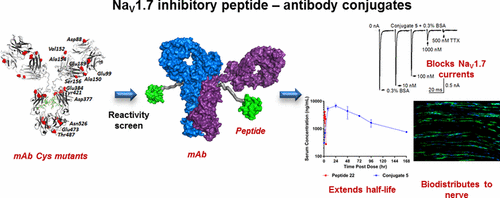
The voltage-gated sodium channel NaV1.7 is a genetically validated pain target under investigation for the development of analgesics. A therapeutic with a less frequent dosing regimen would be of value for treating chronic pain; however functional NaV1.7 targeting antibodies are not known. In this report, we describe NaV1.7 inhibitory peptide–antibody conjugates as an alternate construct for potential prolonged channel blockade through chemical derivatization of engineered antibodies. We previously identified NaV1.7 inhibitory peptide GpTx-1 from tarantula venom and optimized its potency and selectivity. Tethering GpTx-1 peptides to antibodies bifunctionally couples FcRn-based antibody recycling attributes to the NaV1.7 targeting function of the peptide warhead. Herein, we conjugated a GpTx-1 peptide to specific engineered cysteines in a carrier anti-2,4-dinitrophenol monoclonal antibody using polyethylene glycol linkers. The reactivity of 13 potential cysteine conjugation sites in the antibody scaffold was tuned using a model alkylating agent. Subsequent reactions with the peptide identified cysteine locations with the highest conversion to desired conjugates, which blocked NaV1.7 currents in whole cell electrophysiology. Variations in attachment site, linker, and peptide loading established design parameters for potency optimization. Antibody conjugation led to in vivo half-life extension by 130-fold relative to a nonconjugated GpTx-1 peptide and differential biodistribution to nerve fibers in wild-type but not NaV1.7 knockout mice. This study describes the optimization and application of antibody derivatization technology to functionally inhibit NaV1.7 in engineered and neuronal cells.
中文翻译:

工程抗体反应性,可高效衍生以产生Na V 1.7抑制性GpTx-1肽-抗体结合物。
电压门控钠通道Na V 1.7是一种经过遗传验证的止痛目标,正在研究中,用于镇痛药的开发。给药方案频率较低的治疗剂对于治疗慢性疼痛具有重要意义。但是功能性Na V 1.7靶向抗体尚不清楚。在本报告中,我们将Na V 1.7抑制肽-抗体共轭物描述为通过工程化抗体化学衍生化潜在的长时间通道阻断的替代结构。我们先前从狼蛛毒液中鉴定了Na V 1.7抑制肽GpTx-1,并优化了其效价和选择性。将GpTx-1肽与抗体拴系在一起,将基于FcRn的抗体回收属性双功能偶联至Na V1.7肽弹头的靶向功能。本文中,我们使用聚乙二醇接头将GpTx-1肽缀合到载体抗2,4-二硝基苯酚单克隆抗体中的特定工程化半胱氨酸上。使用模型烷基化剂调节抗体支架中13个潜在的半胱氨酸缀合位点的反应性。随后与该肽的反应确定了向所需缀合物转化率最高的半胱氨酸位置,这在全细胞电生理学中阻断了Na V 1.7电流。附着位点,接头和肽负载量的变化为效能优化建立了设计参数。抗体结合导致体内相对于非缀合的GpTx-1肽,其半衰期延长了130倍,并且在野生型小鼠(而非Na V 1.7敲除小鼠)中神经纤维的生物分布差异。这项研究描述了抗体衍生技术的优化及其在功能上抑制工程化和神经元细胞中Na V 1.7的应用。
更新日期:2017-08-29
中文翻译:

工程抗体反应性,可高效衍生以产生Na V 1.7抑制性GpTx-1肽-抗体结合物。
电压门控钠通道Na V 1.7是一种经过遗传验证的止痛目标,正在研究中,用于镇痛药的开发。给药方案频率较低的治疗剂对于治疗慢性疼痛具有重要意义。但是功能性Na V 1.7靶向抗体尚不清楚。在本报告中,我们将Na V 1.7抑制肽-抗体共轭物描述为通过工程化抗体化学衍生化潜在的长时间通道阻断的替代结构。我们先前从狼蛛毒液中鉴定了Na V 1.7抑制肽GpTx-1,并优化了其效价和选择性。将GpTx-1肽与抗体拴系在一起,将基于FcRn的抗体回收属性双功能偶联至Na V1.7肽弹头的靶向功能。本文中,我们使用聚乙二醇接头将GpTx-1肽缀合到载体抗2,4-二硝基苯酚单克隆抗体中的特定工程化半胱氨酸上。使用模型烷基化剂调节抗体支架中13个潜在的半胱氨酸缀合位点的反应性。随后与该肽的反应确定了向所需缀合物转化率最高的半胱氨酸位置,这在全细胞电生理学中阻断了Na V 1.7电流。附着位点,接头和肽负载量的变化为效能优化建立了设计参数。抗体结合导致体内相对于非缀合的GpTx-1肽,其半衰期延长了130倍,并且在野生型小鼠(而非Na V 1.7敲除小鼠)中神经纤维的生物分布差异。这项研究描述了抗体衍生技术的优化及其在功能上抑制工程化和神经元细胞中Na V 1.7的应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号