Catalysis Today ( IF 5.3 ) Pub Date : 2017-08-26 , DOI: 10.1016/j.cattod.2017.08.049 Unseock Kang , Kyu Jun Park , Dong Suk Han , Young-Min Kim , Seungdo Kim , Hyunwoong Park
|
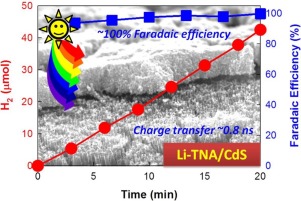
This study reports the synthesis of cadmium sulfide (CdS) nanoparticles on Li+-inserted TiO2 nanotube array (Li-TNA) to fabricate Li-TNA/CdS heterojunction electrodes for photoelectrochemical (PEC) hydrogen production under air mass (AM) 1.5 light and solar visible light (λ > 420 nm). For fabrication of the heterojunction, Li+ is rapidly inserted into TNA pre-grown on Ti foil, and CdS is then photodeposited onto the Li-TNA electrodes for varying deposition times. Surface analyses reveal that sub-100-nm polycrystalline CdS particles partly cover the Li-TNA (length: ∼800 nm, pore diameter: ∼100 nm), enabling the heterojunction to utilize AM 1.5 light as well as visible light. In aqueous solutions of sulfide and sulfite, the Li-TNA/CdS exhibits an incident photon-to-current efficiency (IPCE) of ∼20% (λ = 420 nm) while generating H2 at a Faradaic efficiency of ∼100%. This PEC performance is superior to that of TNA/CdS, which is attributed to the Li+-enhanced charge transfer at the TNA/CdS interface. Electrochemical impedance analysis shows that the charge-transfer resistance of the TNA is reduced by ∼60% by Li+ insertion. Time-resolved photoluminescence decay profiles further reveal that the charge transfer in Li-TNA is completed within 0.8 ns, which is ∼33% faster than that in TNA. The sample surface is analyzed using scanning electron microscopy, X-ray diffraction, energy-dispersive X-ray spectroscopy, X-ray photoelectron spectroscopy, and ultraviolet–visible spectroscopy, and the PEC behavior of the samples is discussed in detail.
中文翻译:

使用光沉积在插入锂离子的二氧化钛纳米管阵列上的CdS纳米粒子进行光电化学制氢
这项研究报告了在Li +插入的TiO 2纳米管阵列(Li-TNA)上合成硫化镉(CdS)纳米颗粒以制造Li-TNA / CdS异质结电极,用于在空气质量(AM)1.5光下产生光电化学(PEC)氢的情况和太阳可见光(λ> 420 nm)。对于异质结的制造,Li +将其快速插入预先在Ti箔上生长的TNA中,然后将CdS光沉积到Li-TNA电极上以改变沉积时间。表面分析表明,低于100 nm的多晶CdS颗粒部分覆盖了Li-TNA(长度:〜800 nm,孔径:〜100 nm),从而使异质结能够利用AM 1.5光和可见光。在硫化物和亚硫酸盐的水溶液中,Li-TNA / CdS表现出约20%(λ= 420 nm)的入射光子-电流效率(IPCE),同时以约100%的法拉第效率产生H 2。该PEC性能优于TNA / CdS,这归因于TNA / CdS接口处Li +增强的电荷转移。电化学阻抗分析表明,Li使TNA的电荷转移电阻降低了约60%+插入。时间分辨的光致发光衰减曲线进一步表明,Li-TNA中的电荷转移在0.8 ns内完成,比TNA中的电荷转移快约33%。使用扫描电子显微镜,X射线衍射,能量色散X射线光谱,X射线光电子光谱和紫外可见光谱对样品表面进行分析,并对样品的PEC行为进行了详细讨论。



























 京公网安备 11010802027423号
京公网安备 11010802027423号