当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Model-Based Analysis of Biopharmaceutic Experiments To Improve Mechanistic Oral Absorption Modeling: An Integrated in Vitro in Vivo Extrapolation Perspective Using Ketoconazole as a Model Drug
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00406 Shriram M. Pathak 1 , Aaron Ruff 2 , Edmund S. Kostewicz 2 , Nikunjkumar Patel 1 , David B. Turner 1 , Masoud Jamei 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00406 Shriram M. Pathak 1 , Aaron Ruff 2 , Edmund S. Kostewicz 2 , Nikunjkumar Patel 1 , David B. Turner 1 , Masoud Jamei 1
Affiliation
|
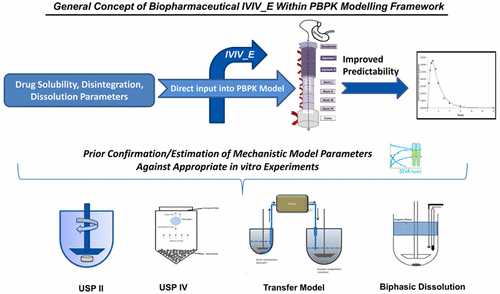
Mechanistic modeling of in vitro data generated from metabolic enzyme systems (viz., liver microsomes, hepatocytes, rCYP enzymes, etc.) facilitates in vitro–in vivo extrapolation (IVIV_E) of metabolic clearance which plays a key role in the successful prediction of clearance in vivo within physiologically-based pharmacokinetic (PBPK) modeling. A similar concept can be applied to solubility and dissolution experiments whereby mechanistic modeling can be used to estimate intrinsic parameters required for mechanistic oral absorption simulation in vivo. However, this approach has not widely been applied within an integrated workflow. We present a stepwise modeling approach where relevant biopharmaceutics parameters for ketoconazole (KTZ) are determined and/or confirmed from the modeling of in vitro experiments before being directly used within a PBPK model. Modeling was applied to various in vitro experiments, namely: (a) aqueous solubility profiles to determine intrinsic solubility, salt limiting solubility factors and to verify pKa; (b) biorelevant solubility measurements to estimate bile-micelle partition coefficients; (c) fasted state simulated gastric fluid (FaSSGF) dissolution for formulation disintegration profiling; and (d) transfer experiments to estimate supersaturation and precipitation parameters. These parameters were then used within a PBPK model to predict the dissolved and total (i.e., including the precipitated fraction) concentrations of KTZ in the duodenum of a virtual population and compared against observed clinical data. The developed model well characterized the intraluminal dissolution, supersaturation, and precipitation behavior of KTZ. The mean simulated AUC0–t of the total and dissolved concentrations of KTZ were comparable to (within 2-fold of) the corresponding observed profile. Moreover, the developed PBPK model of KTZ successfully described the impact of supersaturation and precipitation on the systemic plasma concentration profiles of KTZ for 200, 300, and 400 mg doses. These results demonstrate that IVIV_E applied to biopharmaceutical experiments can be used to understand and build confidence in the quality of the input parameters and mechanistic models used for mechanistic oral absorption simulations in vivo, thereby improving the prediction performance of PBPK models. Moreover, this approach can inform the selection and design of in vitro experiments, potentially eliminating redundant experiments and thus helping to reduce the cost and time of drug product development.
中文翻译:

基于模型的生物药物实验模型分析,以改善机械性口腔吸收模型:使用酮康唑作为模型药物的体内体外推断综合研究
从代谢酶系统(例如,肝微粒体,肝细胞,rCYP酶等)生成的体外数据的机械模型有助于进行代谢清除率的体外-体内外推(IVIV_E),这在成功预测清除率中起关键作用体内基于生理学的药代动力学(PBPK)建模。类似的概念可以应用于溶解度和溶出度实验,从而可以使用机理模型来估算体内机理性口服吸收模拟所需的内在参数。。但是,这种方法尚未在集成工作流中广泛应用。我们提出了一种逐步建模方法,其中在直接用于PBPK模型之前,通过体外实验的建模确定和/或确认了酮康唑(KTZ)的相关生物制药参数。将模型应用于各种体外实验,即:(a)水溶性曲线,以确定固有溶解度,盐限制溶解度因子并验证p K a; (b)与生物有关的溶解度测量,以估计胆胶束分配系数;(c)禁食状态的模拟胃液(FaSSGF)溶出,用于制剂崩解分析;(d)转移实验以估计过饱和度和降水参数。然后,将这些参数用于PBPK模型中,以预测虚拟人群十二指肠中KTZ的溶解浓度和总浓度(即包括沉淀部分),并将其与观察到的临床数据进行比较。开发的模型很好地描述了KTZ的腔内溶解,过饱和和沉淀行为。平均模拟AUC 0– t总的KTZ浓度和溶解的KTZ浓度与相应观察到的曲线相当(在其两倍之内)。此外,已开发的KTZ PBPK模型成功地描述了200、300和400 mg剂量下过饱和和沉淀对KTZ全身血浆浓度分布的影响。这些结果表明,IVIV_E应用于生物药物实验可用于理解和建立用于体内机械口服吸收模拟的输入参数和机械模型的质量的信心,从而提高PBPK模型的预测性能。而且,这种方法可以为体外选择和设计提供参考 实验,有可能消除多余的实验,从而有助于减少药物产品开发的成本和时间。
更新日期:2017-08-26
中文翻译:

基于模型的生物药物实验模型分析,以改善机械性口腔吸收模型:使用酮康唑作为模型药物的体内体外推断综合研究
从代谢酶系统(例如,肝微粒体,肝细胞,rCYP酶等)生成的体外数据的机械模型有助于进行代谢清除率的体外-体内外推(IVIV_E),这在成功预测清除率中起关键作用体内基于生理学的药代动力学(PBPK)建模。类似的概念可以应用于溶解度和溶出度实验,从而可以使用机理模型来估算体内机理性口服吸收模拟所需的内在参数。。但是,这种方法尚未在集成工作流中广泛应用。我们提出了一种逐步建模方法,其中在直接用于PBPK模型之前,通过体外实验的建模确定和/或确认了酮康唑(KTZ)的相关生物制药参数。将模型应用于各种体外实验,即:(a)水溶性曲线,以确定固有溶解度,盐限制溶解度因子并验证p K a; (b)与生物有关的溶解度测量,以估计胆胶束分配系数;(c)禁食状态的模拟胃液(FaSSGF)溶出,用于制剂崩解分析;(d)转移实验以估计过饱和度和降水参数。然后,将这些参数用于PBPK模型中,以预测虚拟人群十二指肠中KTZ的溶解浓度和总浓度(即包括沉淀部分),并将其与观察到的临床数据进行比较。开发的模型很好地描述了KTZ的腔内溶解,过饱和和沉淀行为。平均模拟AUC 0– t总的KTZ浓度和溶解的KTZ浓度与相应观察到的曲线相当(在其两倍之内)。此外,已开发的KTZ PBPK模型成功地描述了200、300和400 mg剂量下过饱和和沉淀对KTZ全身血浆浓度分布的影响。这些结果表明,IVIV_E应用于生物药物实验可用于理解和建立用于体内机械口服吸收模拟的输入参数和机械模型的质量的信心,从而提高PBPK模型的预测性能。而且,这种方法可以为体外选择和设计提供参考 实验,有可能消除多余的实验,从而有助于减少药物产品开发的成本和时间。


























 京公网安备 11010802027423号
京公网安备 11010802027423号