Molecular Cell ( IF 16.0 ) Pub Date : 2017-08-24 , DOI: 10.1016/j.molcel.2017.07.026 Ping Yi 1 , Zhao Wang 2 , Qin Feng 1 , Chao-Kai Chou 3 , Grigore D Pintilie 2 , Hong Shen 1 , Charles E Foulds 1 , Guizhen Fan 4 , Irina Serysheva 4 , Steven J Ludtke 2 , Michael F Schmid 2 , Mien-Chie Hung 3 , Wah Chiu 5 , Bert W O'Malley 1
|
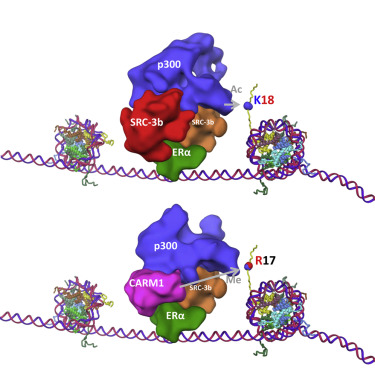
Nuclear receptors recruit multiple coactivators sequentially to activate transcription. This “ordered” recruitment allows different coactivator activities to engage the nuclear receptor complex at different steps of transcription. Estrogen receptor (ER) recruits steroid receptor coactivator-3 (SRC-3) primary coactivator and secondary coactivators, p300/CBP and CARM1. CARM1 recruitment lags behind the binding of SRC-3 and p300 to ER. Combining cryo-electron microscopy (cryo-EM) structure analysis and biochemical approaches, we demonstrate that there is a close crosstalk between early- and late-recruited coactivators. The sequential recruitment of CARM1 not only adds a protein arginine methyltransferase activity to the ER-coactivator complex, it also alters the structural organization of the pre-existing ERE/ERα/SRC-3/p300 complex. It induces a p300 conformational change and significantly increases p300 HAT activity on histone H3K18 residues, which, in turn, promotes CARM1 methylation activity on H3R17 residues to enhance transcriptional activity. This study reveals a structural role for a coactivator sequential recruitment and biochemical process in ER-mediated transcription.
中文翻译:

ER 共激活剂顺序招募的结构和功能影响
核受体依次募集多个共激活因子以激活转录。这种“有序”募集允许不同的共激活剂活动在不同的转录步骤与核受体复合物结合。雌激素受体 (ER) 募集类固醇受体辅激活因子 3 (SRC-3) 初级辅激活因子和次级辅激活因子 p300/CBP 和 CARM1。CARM1 募集滞后于 SRC-3 和 p300 与 ER 的结合。结合冷冻电子显微镜 (cryo-EM) 结构分析和生化方法,我们证明早期和晚期招募的共激活剂之间存在密切的串扰。CARM1 的连续募集不仅为 ER 共激活剂复合物增加了蛋白质精氨酸甲基转移酶活性,还改变了预先存在的 ERE/ERα/SRC-3/p300 复合物的结构组织。它诱导 p300 构象变化并显着增加组蛋白 H3K18 残基上的 p300 HAT 活性,进而促进 H3R17 残基上的 CARM1 甲基化活性以增强转录活性。该研究揭示了共激活剂顺序募集和生化过程在 ER 介导的转录中的结构作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号