当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Placement of Hydroxy Moiety on Pendant of Peptidomimetic Scaffold Modulates Mu and Kappa Opioid Receptor Efficacy
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1021/acschemneuro.7b00284 Aubrie A. Harland 1 , Irina D. Pogozheva 1 , Nicholas W. Griggs 2 , Tyler J. Trask 2 , John R. Traynor 2 , Henry I. Mosberg 1, 3
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1021/acschemneuro.7b00284 Aubrie A. Harland 1 , Irina D. Pogozheva 1 , Nicholas W. Griggs 2 , Tyler J. Trask 2 , John R. Traynor 2 , Henry I. Mosberg 1, 3
Affiliation
|
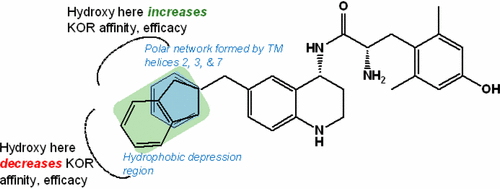
In an effort to expand the structure–activity relationship (SAR) studies of a series of mixed-efficacy opioid ligands, peptidomimetics that incorporate methoxy and hydroxy groups around a benzyl or 2-methylindanyl pendant on a tetrahydroquinoline (THQ) core of the peptidomimetics were evaluated. Compounds containing a methoxy or hydroxy moiety in the o- or m-positions increased binding affinity to the kappa opioid receptor (KOR), whereas compounds containing methoxy or hydroxy groups in the p-position decreased KOR affinity and reduced or eliminated efficacy at the mu opioid receptor (MOR). The results from a substituted 2-methylindanyl series aligned with the findings from the substituted benzyl series. Our studies culminated in the development of 8c, a mixed-efficacy MOR agonist/KOR agonist with subnanomolar binding affinity for both MOR and KOR.
中文翻译:

拟肽支架吊坠上的羟基部分可调节Mu和Kappa阿片受体的功效
为了扩大对一系列混合功效类阿片配体的结构-活性关系(SAR)的研究,拟肽模拟物在拟肽的四氢喹啉(THQ)核心上的苄基或2-甲基茚满基侧基周围结合了甲氧基和羟基。评估。在邻位或间位含有甲氧基或羟基部分的化合物增加了对κ阿片受体(KOR)的结合亲和力,而在对位含有甲氧基或羟基的化合物降低了KOR亲和力并降低或消除了阿片受体(MOR)。取代的2-甲基茚满基系列的结果与取代的苄基系列的结果一致。我们的研究最终导致了图8c是对MOR和KOR具有亚纳摩尔结合亲和力的混合功效的MOR激动剂/ KOR激动剂。
更新日期:2017-08-25
中文翻译:

拟肽支架吊坠上的羟基部分可调节Mu和Kappa阿片受体的功效
为了扩大对一系列混合功效类阿片配体的结构-活性关系(SAR)的研究,拟肽模拟物在拟肽的四氢喹啉(THQ)核心上的苄基或2-甲基茚满基侧基周围结合了甲氧基和羟基。评估。在邻位或间位含有甲氧基或羟基部分的化合物增加了对κ阿片受体(KOR)的结合亲和力,而在对位含有甲氧基或羟基的化合物降低了KOR亲和力并降低或消除了阿片受体(MOR)。取代的2-甲基茚满基系列的结果与取代的苄基系列的结果一致。我们的研究最终导致了图8c是对MOR和KOR具有亚纳摩尔结合亲和力的混合功效的MOR激动剂/ KOR激动剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号