Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Guadecitabine (SGI-110) in treatment-naive patients with acute myeloid leukaemia: phase 2 results from a multicentre, randomised, phase 1/2 trial.
The Lancet ( IF 168.9 ) Pub Date : 2017-10-01 , DOI: 10.1016/s1470-2045(17)30576-4 Hagop M Kantarjian , Gail J Roboz , Patricia L Kropf , Karen W L Yee , Casey L O'Connell , Raoul Tibes , Katherine J Walsh , Nikolai A Podoltsev , Elizabeth A Griffiths , Elias Jabbour , Guillermo Garcia-Manero , David Rizzieri , Wendy Stock , Michael R Savona , Todd L Rosenblat , Jesus G Berdeja , Farhad Ravandi , Edwin P Rock , Yong Hao , Mohammad Azab , Jean-Pierre J Issa
The Lancet ( IF 168.9 ) Pub Date : 2017-10-01 , DOI: 10.1016/s1470-2045(17)30576-4 Hagop M Kantarjian , Gail J Roboz , Patricia L Kropf , Karen W L Yee , Casey L O'Connell , Raoul Tibes , Katherine J Walsh , Nikolai A Podoltsev , Elizabeth A Griffiths , Elias Jabbour , Guillermo Garcia-Manero , David Rizzieri , Wendy Stock , Michael R Savona , Todd L Rosenblat , Jesus G Berdeja , Farhad Ravandi , Edwin P Rock , Yong Hao , Mohammad Azab , Jean-Pierre J Issa
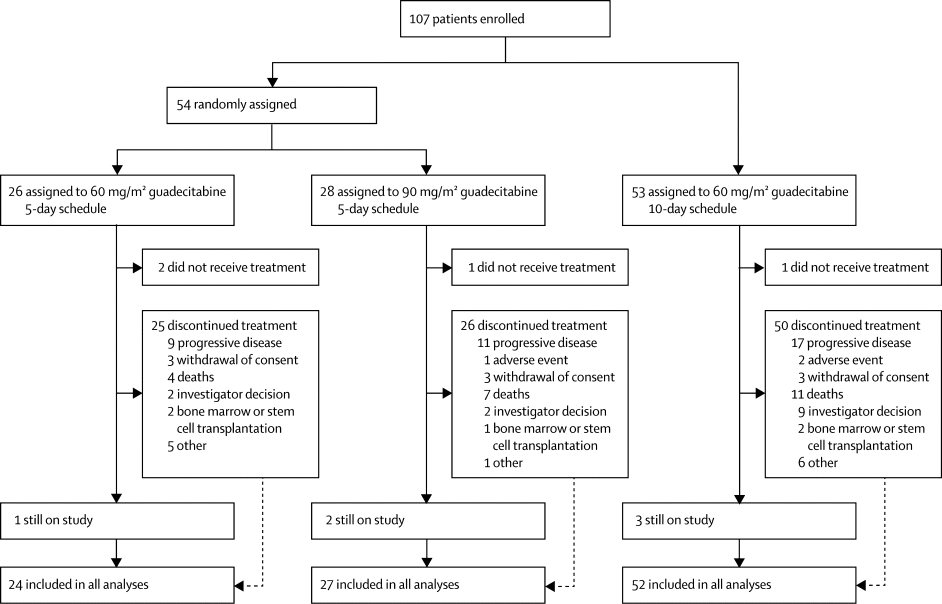
|
The hypomethylating drugs azacitidine and decitabine have shown efficacy in myelodysplastic syndromes and acute myeloid leukaemia, but complete tumour responses are infrequent and of short duration, possibly because of the short half-lives and suboptimal bone marrow exposure of the drugs. Guadecitabine, a next-generation hypomethylating drug, has a longer half-life and exposure than its active metabolite decitabine. A phase 1 study established 60 mg/m2 guadecitabine for 5 days as an effective treatment schedule. In this phase 2 study, we aimed to assess the safety and activity of two doses and schedules of guadecitabine in older (≥65 years) patients with treatment-naive acute myeloid leukaemia who were not candidates for intensive chemotherapy.
中文翻译:

初治急性髓性白血病患者中的瓜地西他滨(SGI-110):第2期来自一项多中心,随机,1/2期试验。
次甲基化药物阿扎胞苷和地西他滨已在骨髓增生异常综合症和急性髓细胞性白血病中显示出疗效,但完整的肿瘤反应并不常见且持续时间短,这可能是由于药物的半衰期短和骨髓暴露不足。瓜地西他滨是下一代次甲基化药物,比其活性代谢物地西他滨具有更长的半衰期和暴露时间。一项1期研究确定60 mg / m 2的guadecitabine有效期为5天。在此2期研究中,我们旨在评估不接受强化化疗的年龄较大(≥65岁)初治急性髓性白血病患者中两次剂量和方案的guadecitabine的安全性和活性。
更新日期:2017-08-25
中文翻译:

初治急性髓性白血病患者中的瓜地西他滨(SGI-110):第2期来自一项多中心,随机,1/2期试验。
次甲基化药物阿扎胞苷和地西他滨已在骨髓增生异常综合症和急性髓细胞性白血病中显示出疗效,但完整的肿瘤反应并不常见且持续时间短,这可能是由于药物的半衰期短和骨髓暴露不足。瓜地西他滨是下一代次甲基化药物,比其活性代谢物地西他滨具有更长的半衰期和暴露时间。一项1期研究确定60 mg / m 2的guadecitabine有效期为5天。在此2期研究中,我们旨在评估不接受强化化疗的年龄较大(≥65岁)初治急性髓性白血病患者中两次剂量和方案的guadecitabine的安全性和活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号