当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Incipient class II mixed valency in a plutonium solid-state compound
Nature Chemistry ( IF 21.8 ) Pub Date : 2017-05-08 00:00:00 , DOI: 10.1038/nchem.2777 Samantha K. Cary , Shane S. Galley , Matthew L. Marsh , David L. Hobart , Ryan E. Baumbach , Justin N. Cross , Jared T. Stritzinger , Matthew J. Polinski , Laurent Maron , Thomas E. Albrecht-Schmitt
Nature Chemistry ( IF 21.8 ) Pub Date : 2017-05-08 00:00:00 , DOI: 10.1038/nchem.2777 Samantha K. Cary , Shane S. Galley , Matthew L. Marsh , David L. Hobart , Ryan E. Baumbach , Justin N. Cross , Jared T. Stritzinger , Matthew J. Polinski , Laurent Maron , Thomas E. Albrecht-Schmitt
|
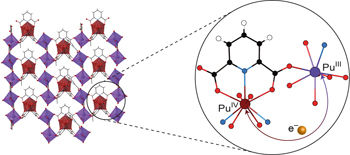
Electron transfer in mixed-valent transition-metal complexes, clusters and materials is ubiquitous in both natural and synthetic systems. The degree to which intervalence charge transfer (IVCT) occurs, dependent on the degree of delocalization, places these within class II or III of the Robin–Day system. In contrast to the d-block, compounds of f-block elements typically exhibit class I behaviour (no IVCT) because of localization of the valence electrons and poor spatial overlap between metal and ligand orbitals. Here, we report experimental and computational evidence for delocalization of 5f electrons in the mixed-valent PuIII/PuIV solid-state compound, Pu3(DPA)5(H2O)2 (DPA = 2,6-pyridinedicarboxylate). The properties of this compound are benchmarked by the pure PuIII and PuIV dipicolinate complexes, [PuIII(DPA)(H2O)4]Br and PuIV(DPA)2(H2O)3·3H2O, as well as by a second mixed-valent compound, PuIII[PuIV(DPA)3H0.5]2, that falls into class I instead. Metal-to-ligand charge transfer is involved in both the formation of Pu3(DPA)5(H2O)2 and in the IVCT.
中文翻译:

a固态化合物中的初始II类混合化合价
混合价过渡金属络合物,簇和材料中的电子转移在天然和合成系统中无处不在。取决于离域化程度,间隔电荷转移(IVCT)发生的程度将其置于Robin-Day系统的II类或III类中。与d-嵌段相反,由于价电子的定位以及金属和配体轨道之间的不良空间重叠,f-嵌段元素的化合物通常表现出I类行为(无IVCT)。在这里,我们报告了5 f电子在混合价Pu III / Pu IV固态化合物Pu 3(DPA)5中离域的实验和计算证据。(H 2 O)2(DPA = 2,6-吡啶二甲酸)。该化合物的性质由纯的Pu III和Pu IV吡啶二羧酸盐络合物[Pu III(DPA)(H 2 O)4 ] Br和Pu IV(DPA)2(H 2 O)3 ·3H 2 O表征,以及第二种混合价化合物Pu III [Pu IV(DPA)3 H 0.5 ] 2,它属于I类。金属到配体的电荷转移参与Pu 3(DPA)的形成5(H 2 O)2和在IVCT中。
更新日期:2017-08-24
中文翻译:

a固态化合物中的初始II类混合化合价
混合价过渡金属络合物,簇和材料中的电子转移在天然和合成系统中无处不在。取决于离域化程度,间隔电荷转移(IVCT)发生的程度将其置于Robin-Day系统的II类或III类中。与d-嵌段相反,由于价电子的定位以及金属和配体轨道之间的不良空间重叠,f-嵌段元素的化合物通常表现出I类行为(无IVCT)。在这里,我们报告了5 f电子在混合价Pu III / Pu IV固态化合物Pu 3(DPA)5中离域的实验和计算证据。(H 2 O)2(DPA = 2,6-吡啶二甲酸)。该化合物的性质由纯的Pu III和Pu IV吡啶二羧酸盐络合物[Pu III(DPA)(H 2 O)4 ] Br和Pu IV(DPA)2(H 2 O)3 ·3H 2 O表征,以及第二种混合价化合物Pu III [Pu IV(DPA)3 H 0.5 ] 2,它属于I类。金属到配体的电荷转移参与Pu 3(DPA)的形成5(H 2 O)2和在IVCT中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号