当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Systemic Delivery of Folate-PEG siRNA Lipopolyplexes with Enhanced Intracellular Stability for In Vivo Gene Silencing in Leukemia
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-08-24 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00383 Dian-Jang Lee,Eva Kessel,Taavi Lehto,Xueying Liu,Naoto Yoshinaga,Kärt Padari,Ying-Chen Chen,Susanne Kempter,Satoshi Uchida,Joachim O. Rädler,Margus Pooga,Ming-Thau Sheu,Kazunori Kataoka,Ernst Wagner
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-08-24 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00383 Dian-Jang Lee,Eva Kessel,Taavi Lehto,Xueying Liu,Naoto Yoshinaga,Kärt Padari,Ying-Chen Chen,Susanne Kempter,Satoshi Uchida,Joachim O. Rädler,Margus Pooga,Ming-Thau Sheu,Kazunori Kataoka,Ernst Wagner
|
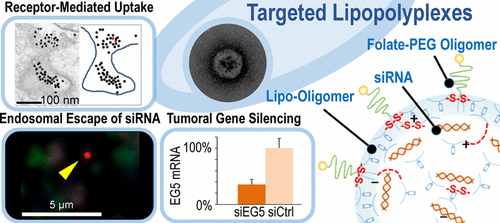
Protection of small interfering RNA (siRNA) against degradation and targeted delivery across the plasma and endosomal membranes to the final site of RNA interference (RNAi) are major aims for the development of siRNA therapeutics. Targeting for folate receptor (FR)-expressing tumors, we optimized siRNA polyplexes by coformulating a folate-PEG-oligoaminoamide (for surface shielding and targeting) with one of three lipo-oligoaminoamides (optionally tyrosine-modified, for optimizing stability and size) to generate ∼100 nm targeted lipopolyplexes (TLPs), which self-stabilize by cysteine disulfide cross-links. To better understand parameters for improved tumor-directed gene silencing, we analyzed intracellular distribution and siRNA release kinetics. FR-mediated endocytosis and endosomal escape of TLPs was confirmed by immuno-TEM. We monitored colocalization of TLPs with endosomes and lysosomes, and onset of siRNA release by time-lapse confocal microscopy; analyzed intracellular stability by FRET using double-labeled siRNA; and correlated results with knockdown of eGFPLuc protein and EG5 mRNA expression. The most potent formulation, TLP1, containing lipopolyplex-stabilizing tyrosine trimers, was found to unpack siRNA in sustained manner with up to 5-fold higher intracellular siRNA stability after 4 h compared to other TLPs. Unexpectedly, data indicated that intracellular siRNA stability instead of an early endosomal exit dominate as a deciding factor for silencing efficiency of TLPs. After i.v. administration in a subcutaneous leukemia mouse model, TLP1 exhibited ligand-dependent tumoral siRNA retention, resulting in 65% EG5 gene silencing at mRNA level without detectable adverse effects. In sum, tyrosine-modified TLP1 conveys superior protection of siRNA for an effective tumor-targeted delivery and RNAi in vivo.
中文翻译:

叶酸-PEG siRNA脂质多聚体的系统递送具有增强的细胞内稳定性,在白血病中体内基因沉默。
保护小分子干扰RNA(siRNA)免受降解,并靶向跨质膜和内体膜到达RNA干扰(RNAi)最终位点的靶向递送,是开发siRNA治疗剂的主要目标。靶向表达叶酸受体(FR)的肿瘤,我们通过将叶酸-PEG-低聚氨基酰胺(用于表面屏蔽和靶向)与三种脂-低聚氨基酰胺之一(可选地,经酪氨酸修饰,以优化稳定性和尺寸)共同配制来优化siRNA复合体,从而产生约100 nm的靶向脂质多聚体(TLP),它们通过半胱氨酸二硫键交联而自我稳定。为了更好地了解改善肿瘤定向基因沉默的参数,我们分析了细胞内分布和siRNA释放动力学。免疫TEM证实了FR介导的TLP的胞吞作用和内体逃逸。我们通过延时共聚焦显微镜监测了TLP与内体和溶酶体的共定位,以及siRNA释放的开始。使用双标记siRNA通过FRET分析细胞内稳定性;并与敲除eGFPLuc蛋白和EG5 mRNA表达相关的结果。与其他TLP相比,发现最有效的制剂TLP1含有可稳定脂多聚体的酪氨酸三聚体,可持续拆封siRNA,并在4小时后将其胞内siRNA稳定性提高了5倍。出乎意料的是,数据表明,细胞内siRNA的稳定性而不是早期的内体退出是决定TLP沉默效率的决定性因素。在皮下白血病小鼠模型中静脉注射后,TLP1表现出依赖配体的肿瘤siRNA保留,导致在mRNA水平上有65%的EG5基因沉默,而没有可检测到的不良影响。总之,酪氨酸修饰的TLP1为有效靶向肿瘤的递送和RNAi提供了siRNA的出色保护。体内。
更新日期:2017-08-24
中文翻译:

叶酸-PEG siRNA脂质多聚体的系统递送具有增强的细胞内稳定性,在白血病中体内基因沉默。
保护小分子干扰RNA(siRNA)免受降解,并靶向跨质膜和内体膜到达RNA干扰(RNAi)最终位点的靶向递送,是开发siRNA治疗剂的主要目标。靶向表达叶酸受体(FR)的肿瘤,我们通过将叶酸-PEG-低聚氨基酰胺(用于表面屏蔽和靶向)与三种脂-低聚氨基酰胺之一(可选地,经酪氨酸修饰,以优化稳定性和尺寸)共同配制来优化siRNA复合体,从而产生约100 nm的靶向脂质多聚体(TLP),它们通过半胱氨酸二硫键交联而自我稳定。为了更好地了解改善肿瘤定向基因沉默的参数,我们分析了细胞内分布和siRNA释放动力学。免疫TEM证实了FR介导的TLP的胞吞作用和内体逃逸。我们通过延时共聚焦显微镜监测了TLP与内体和溶酶体的共定位,以及siRNA释放的开始。使用双标记siRNA通过FRET分析细胞内稳定性;并与敲除eGFPLuc蛋白和EG5 mRNA表达相关的结果。与其他TLP相比,发现最有效的制剂TLP1含有可稳定脂多聚体的酪氨酸三聚体,可持续拆封siRNA,并在4小时后将其胞内siRNA稳定性提高了5倍。出乎意料的是,数据表明,细胞内siRNA的稳定性而不是早期的内体退出是决定TLP沉默效率的决定性因素。在皮下白血病小鼠模型中静脉注射后,TLP1表现出依赖配体的肿瘤siRNA保留,导致在mRNA水平上有65%的EG5基因沉默,而没有可检测到的不良影响。总之,酪氨酸修饰的TLP1为有效靶向肿瘤的递送和RNAi提供了siRNA的出色保护。体内。



























 京公网安备 11010802027423号
京公网安备 11010802027423号