Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2017-08-24 , DOI: 10.1016/j.bmc.2017.08.039 Ahmed M. Gouda , Ahmed H. Abdelazeem , Hany A. Omar , Ashraf N. Abdalla , Mohammed A.S. Abourehab , Hamed I. Ali
|
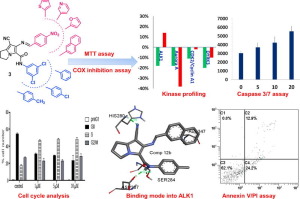
A novel set of pyrrolizine-5-carboxamides has been synthesized and evaluated for their anticancer potential against human breast MCF-7, lung carcinoma A549 and hepatoma Hep3B cancer cell lines. Compound 10c was the most active against MCF-7 with IC50 value of 4.72 µM, while compound 12b was the most active against A549 and Hep3B cell lines. Moreover, kinases/COXs inhibition and apoptosis induction were suggested as potential molecular mechanisms for the anticancer activity of the novel pyrrolizines based on their structural features. The new compounds significantly inhibited COX-1 and COX-2 with IC50 values in the ranges of 5.78–11.96 µM and 0.1–0.78 µM, respectively with high COX-2 selectivity over COX-1. Interestingly, the most potent compound in MTT assay, compound 12b, exhibited high inhibitory activity against COX-2 with selectivity index (COX-1/COX-2) > 100. Meanwhile, compound 12b displayed weak to moderate inhibition of six kinases with inhibition% (7–20%) compared to imatinib (inhibition% = 1–38%). The results of cell cycle analysis, annexin V PI/FITC apoptosis assay and caspase-3/7 assay revealed that compound 12b has the ability to induce apoptosis. The docking results of compound 12b into the active sites of COXs, ALK1 and Aurora kinases indicated that it fits nicely inside their active sites. Overall, the current study highlighted the significant anticancer activity of the newly synthesized pyrrolizines with a potential multi-targeted mechanism which could serve as a base for future studies and further structural optimization into potential anticancer agents.
中文翻译:

吡咯嗪:设计,合成,抗癌评估和潜在作用机理的研究
已经合成了一组新的吡咯烷嗪-5-羧酰胺,并评估了它们对人乳腺MCF-7,肺癌A549和肝癌Hep3B癌细胞系的抗癌潜力。化合物10c对MCF-7最有活性,IC 50值为4.72 µM,而化合物12b对A549和Hep3B细胞系最有活性。此外,激酶/ COXs抑制和凋亡诱导被认为是潜在的分子机制,基于其结构特征的新型吡咯嗪的抗癌活性。新化合物显着抑制具有IC 50的COX-1和COX-2与COX-1相比,COX-2的选择性高,分别在5.78–11.96 µM和0.1–0.78 µM的范围内。有趣的是,MTT分析中最有效的化合物即化合物12b对COX-2表现出高抑制活性,选择性指数(COX-1 / COX-2)>100。同时,化合物12b对6种激酶的抑制作用表现为弱至中度抑制与伊马替尼相比,%(7–20%)(抑制%= 1–38%)。细胞周期分析,膜联蛋白V PI / FITC凋亡测定和caspase-3 / 7测定的结果表明,化合物12b具有诱导细胞凋亡的能力。化合物12b的对接结果在COXs的活性位点中,ALK1和Aurora激酶表明它非常适合它们的活性位点。总的来说,当前的研究强调了新合成的吡咯烷嗪具有显着的抗癌活性,并具有潜在的多靶点作用机制,可作为未来研究和进一步优化潜在抗癌剂结构的基础。


























 京公网安备 11010802027423号
京公网安备 11010802027423号