Journal of Catalysis ( IF 7.3 ) Pub Date : 2017-08-23 , DOI: 10.1016/j.jcat.2017.08.001 Julie Rorrer , Ying He , F. Dean Toste , Alexis T. Bell
|
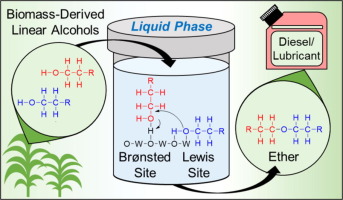
Growing interest in finding renewable alternatives to conventional fossil fuels and petroleum-derived specialty chemicals has motivated the investigation of biomass-derived alcohols to make ethers as diesel additives or lubricants. To optimize the direct etherification of long chain alcohols in the liquid phase, it is necessary to develop an understanding of the kinetics and mechanism of etherification and dehydration reactions. In this study, tungstated zirconia was identified as a selective solid-acid catalyst for the liquid-phase etherification of 1-dodecanol. Investigations of the mechanism and kinetics of this reaction suggest that cooperation between Brønsted- and Lewis-acid sites on tungstated zirconia enhances the selectivity to ether by increasing the surface concentration of adsorbed alcohol, thereby promoting bi-molecular ether formation relative to unimolecular alcohol dehydration. The suggested rate limiting step for etherification is the formation of a CO bond between two adsorbed alcohol molecules, and the suggested rate-limiting step for dehydration is the cleavage of the C
H bond of the β-carbon atom in an adsorbed alcohol. Measurements of the kinetic isotope effects for etherification and dehydration support the proposed mechanism. A microkinetic model based on the proposed mechanism for dodecanol etherification and dehydration over tungstated zirconia accurately describes the observed effects of alcohol concentration and product inhibition.
中文翻译:

钨酸锆上1-十二烷醇醚化的机理和动力学
对寻找常规化石燃料和石油衍生的特种化学品的可再生替代品的兴趣日益浓厚,这促使人们对生物质衍生的醇进行研究,以将醚制成柴油添加剂或润滑剂。为了优化长链醇在液相中的直接醚化,有必要加深对醚化和脱水反应的动力学和机理的了解。在这项研究中,钨酸锆被鉴定为1-十二烷醇液相醚化的选择性固体酸催化剂。对这一反应的机理和动力学的研究表明,钨酸化氧化锆上的布朗斯台德和路易斯酸位点之间的协作通过增加吸附醇的表面浓度来增强对醚的选择性,从而相对于单分子醇脱水促进了双分子醚的形成。建议的醚化速率限制步骤是形成C两个吸附的醇分子之间的O键以及建议的脱水限速步骤是裂解
吸附的醇中β-碳原子的C H键。对醚化和脱水的动力学同位素效应的测量支持了所提出的机理。基于所提出的钨酸化氧化锆上十二烷醇醚化和脱水机理的微动力学模型准确地描述了所观察到的酒精浓度和产物抑制作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号