当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A glutamic acid-modified cellulose fibrous composite used for the adsorption of heavy metal ions from single and binary solutions
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1039/c7qm00210f Meng Li 1, 2, 3, 4 , Zhijiang Liu 1, 2, 3, 4 , Lidong Wang 1, 2, 3, 4 , Tony D. James 5, 6, 7, 8, 9 , Hui-Ning Xiao 1, 2, 3, 4 , Wei-Hong Zhu 10, 11, 12, 13, 14
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1039/c7qm00210f Meng Li 1, 2, 3, 4 , Zhijiang Liu 1, 2, 3, 4 , Lidong Wang 1, 2, 3, 4 , Tony D. James 5, 6, 7, 8, 9 , Hui-Ning Xiao 1, 2, 3, 4 , Wei-Hong Zhu 10, 11, 12, 13, 14
Affiliation
|
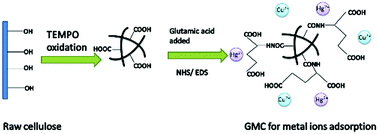
A new glutamic acid-modified cellulose was prepared and investigated as a bioadsorbent used for the simple and rapid removal of Cu2+ and Hg2+ from single and binary aqueous solutions. The effects of the initial concentration of heavy metal ions, pH of the solution and temperature on the adsorption capacity of the bioadsorbent were investigated. Equilibrium studies demonstrate that the adsorption of Cu2+ and Hg2+ follows the Langmuir model in a single aqueous solution system. Meanwhile, the adsorption kinetics were found to follow the pseudo-second-order model well. Competitive adsorption in the binary component system indicates the mutual inhibitation adsorption between the two metal ions. The FTIR and SEM–EDS results reveal that Cu2+ and Hg2+ are successfully adsorbed on the bioadsorbent by coordination and static interactions.
中文翻译:

谷氨酸改性的纤维素纤维复合材料,用于从单一溶液和二元溶液中吸附重金属离子
制备了一种新的谷氨酸修饰的纤维素,并将其用作生物吸附剂,用于从单一和二元水溶液中简单,快速地去除Cu 2+和Hg 2+。研究了重金属离子的初始浓度,溶液的pH值和温度对生物吸附剂吸附能力的影响。平衡研究表明,在单一水溶液系统中,Cu 2+和Hg 2+的吸附遵循Langmuir模型。同时,发现吸附动力学很好地遵循了伪二级模型。二元组分系统中的竞争性吸附表明两种金属离子之间的相互抑制吸附。FTIR和SEM-EDS结果表明,铜2+和Hg 2+通过配位和静态相互作用成功吸附在生物吸附剂上。
更新日期:2017-08-24
中文翻译:

谷氨酸改性的纤维素纤维复合材料,用于从单一溶液和二元溶液中吸附重金属离子
制备了一种新的谷氨酸修饰的纤维素,并将其用作生物吸附剂,用于从单一和二元水溶液中简单,快速地去除Cu 2+和Hg 2+。研究了重金属离子的初始浓度,溶液的pH值和温度对生物吸附剂吸附能力的影响。平衡研究表明,在单一水溶液系统中,Cu 2+和Hg 2+的吸附遵循Langmuir模型。同时,发现吸附动力学很好地遵循了伪二级模型。二元组分系统中的竞争性吸附表明两种金属离子之间的相互抑制吸附。FTIR和SEM-EDS结果表明,铜2+和Hg 2+通过配位和静态相互作用成功吸附在生物吸附剂上。



























 京公网安备 11010802027423号
京公网安备 11010802027423号