当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Degradation of ibuprofen using ozone combined with peroxymonosulfate
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2017-08-01 00:00:00 , DOI: 10.1039/c7ew00174f Zhen Yuan 1, 2, 3, 4, 5 , Minghao Sui 1, 2, 3, 4, 5 , Bojie Yuan 1, 2, 3, 4, 5 , Pan Li 1, 2, 3, 4, 5 , Jingyu Wang 1, 2, 3, 4, 5 , Jie Qin 1, 2, 3, 4, 5 , Guangyi Xu 1, 2, 3, 4, 5
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2017-08-01 00:00:00 , DOI: 10.1039/c7ew00174f Zhen Yuan 1, 2, 3, 4, 5 , Minghao Sui 1, 2, 3, 4, 5 , Bojie Yuan 1, 2, 3, 4, 5 , Pan Li 1, 2, 3, 4, 5 , Jingyu Wang 1, 2, 3, 4, 5 , Jie Qin 1, 2, 3, 4, 5 , Guangyi Xu 1, 2, 3, 4, 5
Affiliation
|
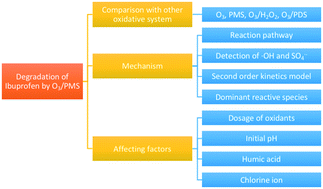
Ozone combined with peroxymonosulfate (O3/PMS) for degradation of ibuprofen (IBP) was studied in this work. The oxidation capacity of O3/PMS and four other related oxidation processes for IBP degradation was compared. O3/PMS demonstrated a compelling degradation ability towards IBP compared with O3, PMS, O3/PDS, and O3/H2O2 processes. The generation of ˙OH and SO4˙− in O3/PMS was proved by electron spin resonance detection, and the dominant highly oxidative species was determined based on the different scavenging performances of tert-butanol and ethanol towards ˙OH and SO4˙−. The affecting factors including O3 and PMS dosage, initial pH, and organic and inorganic constituents in water were investigated. An optimum dosage of O3 and PMS was observed for degradation of IBP in O3/PMS. Though a notably synergistic effect was observed in O3/PMS for IBP degradation under pH 3.0, 5.0, 7.6 and 9.0, alkaline conditions were found to be more beneficial for IBP degradation. Humic acid, which was used to simulate the natural organic water matrix, posed different effects on O3 and O3/PMS for IBP degradation. The presence of Cl−, which is a typical reductive inorganic ion and ubiquitous in natural water, inhibited O3/PMS oxidation on the degradation of IBP. The degradation kinetics of IBP in O3/PMS was also studied in this work.
中文翻译:

臭氧与过氧一硫酸盐联合降解布洛芬
在这项工作中,研究了臭氧与过氧一硫酸盐(O 3 / PMS)组合用于降解布洛芬(IBP)的问题。比较了O 3 / PMS的氧化能力和其他四个有关IBP降解的氧化过程。与O 3,PMS,O 3 / PDS和O 3 / H 2 O 2工艺相比,O 3 / PMS表现出令人信服的IBP降解能力。OH的生成和SO 4 ˙ -为O 3 / PMS通过电子自旋共振检测证明,以及基于所述不同的清除性能被确定的主导高度氧化性物质叔丁醇和朝向OH乙醇和SO 4 ˙ - 。研究了影响O 3和PMS用量,初始pH值以及水中有机和无机成分的因素。观察到最佳的O 3和PMS剂量可降解O 3 / PMS中的IBP 。尽管在O 3 / PMS中观察到在pH 3.0、5.0、7.6和9.0下IBP降解具有明显的协同作用,但发现碱性条件对IBP降解更有利。用于模拟天然有机水基质的腐殖酸对O 3和O 3 / PMS降解IBP的影响不同。氯的存在-是一种典型的还原性无机离子,在天然水中普遍存在,可抑制O 3 / PMS对IBP降解的氧化。这项工作还研究了IBP在O 3 / PMS中的降解动力学。
更新日期:2017-08-24
中文翻译:

臭氧与过氧一硫酸盐联合降解布洛芬
在这项工作中,研究了臭氧与过氧一硫酸盐(O 3 / PMS)组合用于降解布洛芬(IBP)的问题。比较了O 3 / PMS的氧化能力和其他四个有关IBP降解的氧化过程。与O 3,PMS,O 3 / PDS和O 3 / H 2 O 2工艺相比,O 3 / PMS表现出令人信服的IBP降解能力。OH的生成和SO 4 ˙ -为O 3 / PMS通过电子自旋共振检测证明,以及基于所述不同的清除性能被确定的主导高度氧化性物质叔丁醇和朝向OH乙醇和SO 4 ˙ - 。研究了影响O 3和PMS用量,初始pH值以及水中有机和无机成分的因素。观察到最佳的O 3和PMS剂量可降解O 3 / PMS中的IBP 。尽管在O 3 / PMS中观察到在pH 3.0、5.0、7.6和9.0下IBP降解具有明显的协同作用,但发现碱性条件对IBP降解更有利。用于模拟天然有机水基质的腐殖酸对O 3和O 3 / PMS降解IBP的影响不同。氯的存在-是一种典型的还原性无机离子,在天然水中普遍存在,可抑制O 3 / PMS对IBP降解的氧化。这项工作还研究了IBP在O 3 / PMS中的降解动力学。


























 京公网安备 11010802027423号
京公网安备 11010802027423号