Molecular Cell ( IF 16.0 ) Pub Date : 2017-07-27 , DOI: 10.1016/j.molcel.2017.06.033 Sang-Oh Yoon 1 , Sejeong Shin 1 , Florian A Karreth 2 , Gwen R Buel 1 , Mark P Jedrychowski 3 , David R Plas 4 , Shoukat Dedhar 5 , Steven P Gygi 3 , Philippe P Roux 6 , Noah Dephoure 7 , John Blenis 1
|
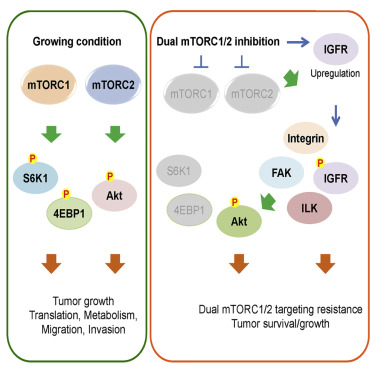
Aberrant signaling by the mammalian target of rapamycin (mTOR) contributes to the devastating features of cancer cells. Thus, mTOR is a critical therapeutic target and catalytic inhibitors are being investigated as anti-cancer drugs. Although mTOR inhibitors initially block cell proliferation, cell viability and migration in some cancer cells are quickly restored. Despite sustained inhibition of mTORC1/2 signaling, Akt, a kinase regulating cell survival and migration, regains phosphorylation at its regulatory sites. Mechanistically, mTORC1/2 inhibition promotes reorganization of integrin/focal adhesion kinase-mediated adhesomes, induction of IGFR/IR-dependent PI3K activation, and Akt phosphorylation via an integrin/FAK/IGFR-dependent process. This resistance mechanism contributes to xenograft tumor cell growth, which is prevented with mTOR plus IGFR inhibitors, supporting this combination as a therapeutic approach for cancers.
中文翻译:

局灶性粘附和IGF1R依赖的生存和迁移途径介导肿瘤对mTORC1 / 2抑制的抵抗力。
雷帕霉素(mTOR)的哺乳动物靶标引起的异常信号转导导致癌细胞的破坏性特征。因此,mTOR是关键的治疗靶标,并且催化抑制剂正在被研究作为抗癌药物。尽管mTOR抑制剂起初会阻止细胞增殖,但某些癌细胞中的细胞活力和迁移会迅速恢复。尽管持续抑制mTORC1 / 2信号传导,但调节细胞存活和迁移的激酶Akt在其调节位点恢复了磷酸化。从机制上讲,mTORC1 / 2抑制通过整联蛋白/ FAK / IGFR依赖性过程促进整联蛋白/局灶性粘附激酶介导的脂质体的重组,诱导IGFR / IR依赖性PI3K活化和Akt磷酸化。这种抗性机制有助于异种移植肿瘤细胞的生长,



























 京公网安备 11010802027423号
京公网安备 11010802027423号