当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficacy of Rosuvastatin in Children With Homozygous Familial Hypercholesterolemia and Association With Underlying Genetic Mutations
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2017-08-01 , DOI: 10.1016/j.jacc.2017.06.058 Evan A. Stein , Eldad J. Dann , Albert Wiegman , Flemming Skovby , Daniel Gaudet , Etienne Sokal , Min-Ji Charng , Mafauzy Mohamed , Ilse Luirink , Joel S. Raichlen , Mattias Sundén , Stefan C. Carlsson , Frederick J. Raal , John J.P. Kastelein
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2017-08-01 , DOI: 10.1016/j.jacc.2017.06.058 Evan A. Stein , Eldad J. Dann , Albert Wiegman , Flemming Skovby , Daniel Gaudet , Etienne Sokal , Min-Ji Charng , Mafauzy Mohamed , Ilse Luirink , Joel S. Raichlen , Mattias Sundén , Stefan C. Carlsson , Frederick J. Raal , John J.P. Kastelein
|
BACKGROUND
Homozygous familial hypercholesterolemia (HoFH), a rare genetic disorder, is characterized by extremely elevated levels of low-density lipoprotein cholesterol (LDL-C) and accelerated atherosclerotic cardiovascular disease. Statin treatment starts at diagnosis, but no statin has been formally evaluated in, or approved for, HoFH children. OBJECTIVES
The authors sought to assess the LDL-C efficacy of rosuvastatin versus placebo in HoFH children, and the relationship with underlying genetic mutations. METHODS
This was a randomized, double-blind, 12-week, crossover study of rosuvastatin 20 mg versus placebo, followed by 12 weeks of open-label rosuvastatin. Patients discontinued all lipid-lowering treatment except ezetimibe and/or apheresis. Clinical and laboratory assessments were performed every 6 weeks. The relationship between LDL-C response and genetic mutations was assessed by adding children and adults from a prior HoFH rosuvastatin trial. RESULTS
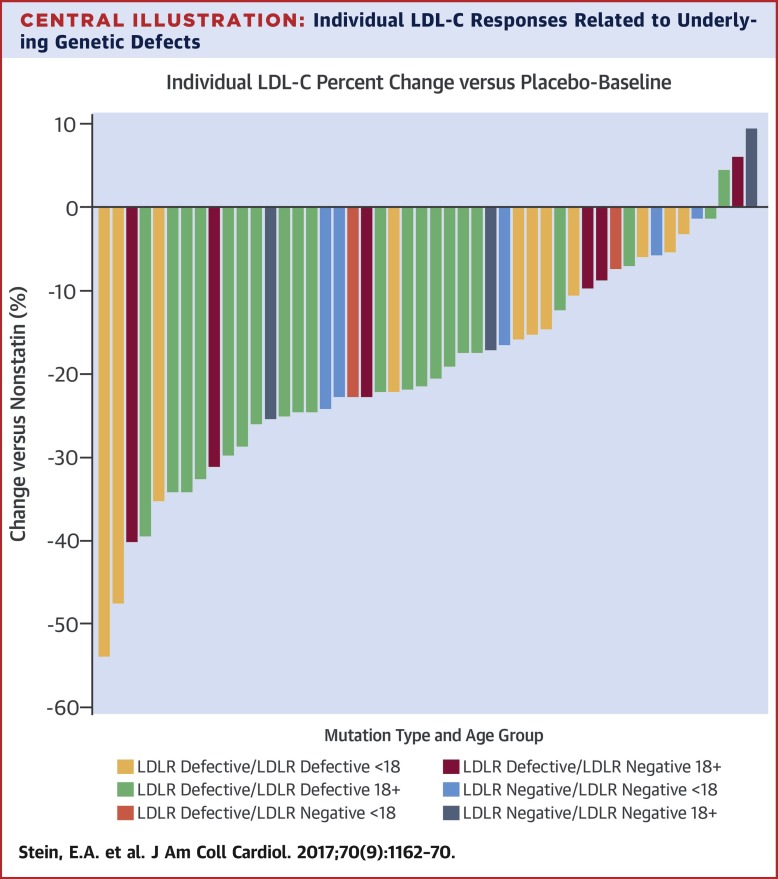
Twenty patients were screened, 14 randomized, and 13 completed the study. The mean age was 10.9 years; 8 patients were on ezetimibe and 7 on apheresis. Mean LDL-C was 481 mg/dl (range: 229 to 742 mg/dl) on placebo and 396 mg/dl (range: 130 to 700 mg/dl) on rosuvastatin, producing a mean 85.4 mg/dl (22.3%) difference (p = 0.005). Efficacy was similar regardless of age or use of ezetimibe or apheresis, and was maintained for 12 weeks. Adverse events were few and not serious. Patients with 2 defective versus 2 negative LDL receptor mutations had mean LDL-C reductions of 23.5% (p = 0.0044) and 14% (p = 0.038), respectively. CONCLUSIONS
This first-ever pediatric HoFH statin trial demonstrated safe and effective LDL-C reduction with rosuvastatin 20 mg alone or added to ezetimibe and/or apheresis. The LDL-C response in children and adults was related to underlying genetic mutations. (A Study to Evaluate the Efficacy and Safety of Rosuvastatin in Children and Adolescents With Homozygous Familial Hypercholesterolemia [HYDRA]; NCT02226198).
中文翻译:

瑞舒伐他汀对纯合子家族性高胆固醇血症儿童的疗效及其与潜在遗传突变的关联
背景纯合子家族性高胆固醇血症 (HoFH) 是一种罕见的遗传疾病,其特征是低密度脂蛋白胆固醇 (LDL-C) 水平极度升高和加速动脉粥样硬化性心血管疾病。他汀类药物治疗从诊断开始,但尚未正式评估或批准用于 HoFH 儿童的他汀类药物。目的 作者试图评估瑞舒伐他汀与安慰剂在 HoFH 儿童中的 LDL-C 疗效,以及与潜在基因突变的关系。方法 这是一项随机、双盲、12 周、瑞舒伐他汀 20 毫克与安慰剂的交叉研究,随后是 12 周的开放标签瑞舒伐他汀。除依折麦布和/或单采术外,患者停止了所有降脂治疗。每 6 周进行一次临床和实验室评估。通过将先前 HoFH 瑞舒伐他汀试验中的儿童和成人加入进来,评估了 LDL-C 反应与基因突变之间的关系。结果 筛选了 20 名患者,随机分配了 14 名,13 名完成了研究。平均年龄为 10.9 岁;8 名患者使用依折麦布,7 名患者使用单采。平均 LDL-C 安慰剂组为 481 mg/dl(范围:229 至 742 mg/dl),瑞舒伐他汀组为 396 mg/dl(范围:130 至 700 mg/dl),产生平均 85.4 mg/dl (22.3%)差异(p = 0.005)。无论年龄或使用依折麦布或单采术,疗效均相似,并维持 12 周。不良事件很少,也不严重。具有 2 个缺陷和 2 个阴性 LDL 受体突变的患者的平均 LDL-C 降低分别为 23.5% (p = 0.0044) 和 14% (p = 0.038)。结论 这项首个儿科 HoFH 他汀类药物试验证明,单独使用瑞舒伐他汀 20 mg 或添加到依折麦布和/或单采术中可安全有效地降低 LDL-C。儿童和成人的 LDL-C 反应与潜在的基因突变有关。(一项评估瑞舒伐他汀对纯合子家族性高胆固醇血症 [HYDRA] 儿童和青少年的疗效和安全性的研究;NCT02226198)。
更新日期:2017-08-01
中文翻译:

瑞舒伐他汀对纯合子家族性高胆固醇血症儿童的疗效及其与潜在遗传突变的关联
背景纯合子家族性高胆固醇血症 (HoFH) 是一种罕见的遗传疾病,其特征是低密度脂蛋白胆固醇 (LDL-C) 水平极度升高和加速动脉粥样硬化性心血管疾病。他汀类药物治疗从诊断开始,但尚未正式评估或批准用于 HoFH 儿童的他汀类药物。目的 作者试图评估瑞舒伐他汀与安慰剂在 HoFH 儿童中的 LDL-C 疗效,以及与潜在基因突变的关系。方法 这是一项随机、双盲、12 周、瑞舒伐他汀 20 毫克与安慰剂的交叉研究,随后是 12 周的开放标签瑞舒伐他汀。除依折麦布和/或单采术外,患者停止了所有降脂治疗。每 6 周进行一次临床和实验室评估。通过将先前 HoFH 瑞舒伐他汀试验中的儿童和成人加入进来,评估了 LDL-C 反应与基因突变之间的关系。结果 筛选了 20 名患者,随机分配了 14 名,13 名完成了研究。平均年龄为 10.9 岁;8 名患者使用依折麦布,7 名患者使用单采。平均 LDL-C 安慰剂组为 481 mg/dl(范围:229 至 742 mg/dl),瑞舒伐他汀组为 396 mg/dl(范围:130 至 700 mg/dl),产生平均 85.4 mg/dl (22.3%)差异(p = 0.005)。无论年龄或使用依折麦布或单采术,疗效均相似,并维持 12 周。不良事件很少,也不严重。具有 2 个缺陷和 2 个阴性 LDL 受体突变的患者的平均 LDL-C 降低分别为 23.5% (p = 0.0044) 和 14% (p = 0.038)。结论 这项首个儿科 HoFH 他汀类药物试验证明,单独使用瑞舒伐他汀 20 mg 或添加到依折麦布和/或单采术中可安全有效地降低 LDL-C。儿童和成人的 LDL-C 反应与潜在的基因突变有关。(一项评估瑞舒伐他汀对纯合子家族性高胆固醇血症 [HYDRA] 儿童和青少年的疗效和安全性的研究;NCT02226198)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号