当前位置:
X-MOL 学术
›
Mol. Biosyst.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rational design of an orthogonal noncovalent interaction system at the MUPP1 PDZ11 complex interface with CaMKIIα-derived peptides in human fertilization
Molecular BioSystems Pub Date : 2017-07-21 00:00:00 , DOI: 10.1039/c7mb00379j Yi-Le Zhang 1, 2, 3, 4 , Zhao-Feng Han 2, 3, 4, 5
Molecular BioSystems Pub Date : 2017-07-21 00:00:00 , DOI: 10.1039/c7mb00379j Yi-Le Zhang 1, 2, 3, 4 , Zhao-Feng Han 2, 3, 4, 5
Affiliation
|
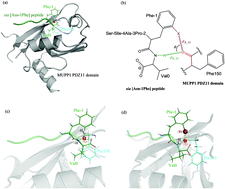
The recognition and association between the Ca2+/calmodulin-activated protein kinase II-α (CaMKIIα) and the multi-PDZ domain protein 1 (MUPP1) plays an important role in the sperm acrosome reaction and human fertilization. Previously, we have demonstrated that the MUPP1 PDZ11 domain is the primary binding partner of the CaMKIIα C-terminal tail, which can be targeted by a rationally designed sia peptide with nanomolar affinity. Here, we further introduced an orthogonal noncovalent interaction (ONI) system between a native hydrogen bond and a designed halogen bond across the complex interface of the PDZ11 domain with the sia [Asn-1Phe] peptide mutant, where the halogen bond was formed by substituting the o-hydrogen atom of the benzene ring of the peptide Phe-1 residue with a halogen atom (F, Cl, Br or I). Molecular dynamics simulations and high-level theoretical calculations suggested that bromine (Br) is a good compromise between the halogen-bonding strength and steric hindrance effect due to introduction of a bulkier halogen atom into the tightly packed complex interface. Fluorescence spectroscopy assays revealed that the resulting o-Br-substituted peptide (Kd = 18 nM) exhibited an ∼7.6-fold affinity increase relative to its native counterpart (Kd = 137 nM). In contrast, the p-Br-substituted peptide, a negative control that is unable to establish the ONI according to structure-based analysis, has decreased affinity (Kd = 210 nM) upon halogenation.
中文翻译:

MUPP1 PDZ11复合体与CaMKIIα衍生肽在人受精中的正交非共价相互作用系统的合理设计
Ca 2+ /钙调蛋白激活的蛋白激酶II-α(CaMKIIα)和多PDZ域蛋白1(MUPP1)之间的识别和关联在精子顶体反应和人类受精中起重要作用。以前,我们已经证明MUPP1 PDZ11域是CaMKIIαC末端尾巴的主要结合配偶体,可以通过合理设计的具有纳摩尔摩尔亲和力的sia肽作为靶向。在这里,我们进一步介绍了PDZ11域与sia [Asn-1Phe]肽突变体的复杂界面之间的天然氢键与设计的卤素键之间的正交非共价相互作用(ONI)系统,其中的卤素键是通过取代形成的该Ø肽Phe-1残基的苯环的-氢原子具有卤素原子(F,Cl,Br或I)。分子动力学模拟和高级理论计算表明,由于将大量的卤原子引入紧密堆积的复杂界面,因此溴(Br)是卤素键强度和位阻效应之间的良好折衷。荧光光谱分析表明,相对于其天然对应物(K d = 137 nM),所生成的邻-Br取代肽(K d = 18 nM)显示出约7.6倍的亲和力增加。相反,p-Br-取代的肽,根据基于结构的分析无法建立ONI的阴性对照,卤化后亲和力降低(K d = 210 nM)。
更新日期:2017-08-23
中文翻译:

MUPP1 PDZ11复合体与CaMKIIα衍生肽在人受精中的正交非共价相互作用系统的合理设计
Ca 2+ /钙调蛋白激活的蛋白激酶II-α(CaMKIIα)和多PDZ域蛋白1(MUPP1)之间的识别和关联在精子顶体反应和人类受精中起重要作用。以前,我们已经证明MUPP1 PDZ11域是CaMKIIαC末端尾巴的主要结合配偶体,可以通过合理设计的具有纳摩尔摩尔亲和力的sia肽作为靶向。在这里,我们进一步介绍了PDZ11域与sia [Asn-1Phe]肽突变体的复杂界面之间的天然氢键与设计的卤素键之间的正交非共价相互作用(ONI)系统,其中的卤素键是通过取代形成的该Ø肽Phe-1残基的苯环的-氢原子具有卤素原子(F,Cl,Br或I)。分子动力学模拟和高级理论计算表明,由于将大量的卤原子引入紧密堆积的复杂界面,因此溴(Br)是卤素键强度和位阻效应之间的良好折衷。荧光光谱分析表明,相对于其天然对应物(K d = 137 nM),所生成的邻-Br取代肽(K d = 18 nM)显示出约7.6倍的亲和力增加。相反,p-Br-取代的肽,根据基于结构的分析无法建立ONI的阴性对照,卤化后亲和力降低(K d = 210 nM)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号