当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
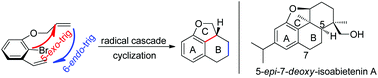
Rapid construction of the 6/6/5 tricyclic framework via a tandem radical cyclization reaction and its application to the synthesis of 5-epi-7-deoxy-isoabietenin A
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1039/c7qo00550d Hao Zhang 1, 2, 3, 4, 5 , Shiqiang Ma 1, 2, 3, 4, 5 , Zhimin Xing 1, 2, 3, 4, 5 , Lin Liu 1, 2, 3, 4, 5 , Bowen Fang 5, 6, 7, 8 , Xingang Xie 1, 2, 3, 4, 5 , Xuegong She 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1039/c7qo00550d Hao Zhang 1, 2, 3, 4, 5 , Shiqiang Ma 1, 2, 3, 4, 5 , Zhimin Xing 1, 2, 3, 4, 5 , Lin Liu 1, 2, 3, 4, 5 , Bowen Fang 5, 6, 7, 8 , Xingang Xie 1, 2, 3, 4, 5 , Xuegong She 1, 2, 3, 4, 5
Affiliation
|
A tandem radical cyclization reaction towards the 6/6/5 fused tricyclic skeleton, which exists in numerous natural products, was developed in modest to good yields. In this transformation, two C–C bonds and two rings were formed successively via a tandem 5-exo-trig/6-endo-trig cyclization reaction. 5-epi-7-deoxy-Isoabietenin A was also synthesized efficiently via this strategy.
中文翻译:

通过串联自由基环化反应快速构建6/6/5三环骨架,并将其应用于5- epi -7-deoxy-isoabietenin A的合成
大量存在于天然产物中的向6/6/5稠合三环骨架的串联自由基环化反应已适度提高到了良好的收率。在这种转化中,通过串联的5- exo- trig / 6- endo- trig环化反应连续形成两个CC键和两个环。通过这种策略也有效地合成了5- epi -7-脱氧-异豆蛋白A。
更新日期:2017-08-23
中文翻译:

通过串联自由基环化反应快速构建6/6/5三环骨架,并将其应用于5- epi -7-deoxy-isoabietenin A的合成
大量存在于天然产物中的向6/6/5稠合三环骨架的串联自由基环化反应已适度提高到了良好的收率。在这种转化中,通过串联的5- exo- trig / 6- endo- trig环化反应连续形成两个CC键和两个环。通过这种策略也有效地合成了5- epi -7-脱氧-异豆蛋白A。



























 京公网安备 11010802027423号
京公网安备 11010802027423号