当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Process exploration and assessment for the production of methanol and dimethyl ether from carbon dioxide and water
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1039/c7se00206h A. Hankin 1, 2, 3, 4 , N. Shah 1, 2, 3, 4
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1039/c7se00206h A. Hankin 1, 2, 3, 4 , N. Shah 1, 2, 3, 4
Affiliation
|
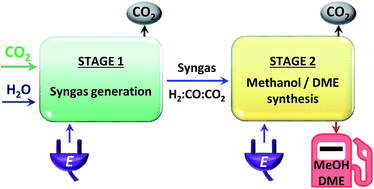
A thermodynamic, model-based, study was carried out to assess the relative performance of methanol and dimethyl ether (DME) synthesis systems using CO- and CO2-based syngas feeds. The upstream production of a range of syngas feed compositions was simulated using CO2 and H2O as the sole chemical building blocks, a requirement motivated by the increasing constraints on permissible CO2 emissions and the successful adaptation by some industrial methanol plants to the direct utilisation of CO2. The objective was to establish whether the energy requirements and CO2 emissions associated with upstream conversion of CO2 to CO were justified by increased productivity in the methanol/DME systems. In the first part of the study, the performance of four systems was evaluated and compared in terms of energy efficiency and CO2 conversion: (1) methanol synthesis system, (2) direct DME synthesis system, (3) two-step DME synthesis system with an interposed syngas separation step between the methanol production reactor and methanol dehydration reactor and (4) two-step DME synthesis system with no separation step between the two reactors. Based on equilibrium yields at 250 °C and 50 bar, the direct DME synthesis system was found to exhibit the highest energy conversion efficiencies with both CO2- and CO-based syngas. Although this system demonstrated the lowest CO2 emissions per methanol equivalent product with a CO-based feed, the benefits were offset by emissions associated with the upstream conversion of H2O and CO2 to H2 and CO, evaluated in the second part of the study. It was determined that CO2 could be utilised directly in the direct DME synthesis route, whereas upstream conversion of CO2 to CO was necessary to achieve effective yields in the methanol/two-step DME systems. CO-based syngas production via high temperature co-electrolysis of H2O and CO2, or alternatively high temperature CO2 electrolysis coupled with the water–gas shift process, was identified as the best technology based on energy consumption and CO2 utilisation.
中文翻译:

由二氧化碳和水生产甲醇和二甲醚的工艺探索和评估
进行了基于模型的热力学研究,以评估使用基于CO和CO 2的合成气进料的甲醇和二甲醚(DME)合成系统的相对性能。使用CO 2和H 2 O作为唯一的化学组成部分模拟了一系列合成气进料组成的上游生产,这一要求是由于对可允许的CO 2排放的限制日益严格以及一些工业甲醇工厂成功地适应了直接利用CO 2。目的是确定与上游转化的CO 2是否相关的能源需求和CO 2排放在甲醇/ DME系统中提高生产率证明了二氧化碳转化为二氧化碳的合理性。在研究的第一部分中,对四个系统的性能进行了评估,并就能量效率和CO 2转化进行了比较:(1)甲醇合成系统,(2)直接DME合成系统,(3)两步DME合成该系统具有在甲醇生产反应器和甲醇脱水反应器之间插入的合成气分离步骤和(4)两步DME合成系统,在两个反应器之间没有分离步骤。基于在250°C和50 bar下的平衡产率,发现直接DME合成系统在CO 2和CO基合成气中均显示出最高的能量转换效率。尽管该系统显示出最低的CO 2每一个基于CO-进料甲醇当量产物的排放量,好处通过用H的上游转化相关联的排放量偏移2 O和CO 2与H 2和CO,在研究的第二部分进行评价。已确定可以在直接DME合成路线中直接利用CO 2,而在甲醇/两步DME系统中将CO 2上游转化为CO是实现有效收率所必需的。通过H 2 O和CO 2或高温CO 2的高温共电解生产基于CO的合成气基于能耗和CO 2利用,电解与水煤气变换过程相结合被认为是最好的技术。
更新日期:2017-08-23
中文翻译:

由二氧化碳和水生产甲醇和二甲醚的工艺探索和评估
进行了基于模型的热力学研究,以评估使用基于CO和CO 2的合成气进料的甲醇和二甲醚(DME)合成系统的相对性能。使用CO 2和H 2 O作为唯一的化学组成部分模拟了一系列合成气进料组成的上游生产,这一要求是由于对可允许的CO 2排放的限制日益严格以及一些工业甲醇工厂成功地适应了直接利用CO 2。目的是确定与上游转化的CO 2是否相关的能源需求和CO 2排放在甲醇/ DME系统中提高生产率证明了二氧化碳转化为二氧化碳的合理性。在研究的第一部分中,对四个系统的性能进行了评估,并就能量效率和CO 2转化进行了比较:(1)甲醇合成系统,(2)直接DME合成系统,(3)两步DME合成该系统具有在甲醇生产反应器和甲醇脱水反应器之间插入的合成气分离步骤和(4)两步DME合成系统,在两个反应器之间没有分离步骤。基于在250°C和50 bar下的平衡产率,发现直接DME合成系统在CO 2和CO基合成气中均显示出最高的能量转换效率。尽管该系统显示出最低的CO 2每一个基于CO-进料甲醇当量产物的排放量,好处通过用H的上游转化相关联的排放量偏移2 O和CO 2与H 2和CO,在研究的第二部分进行评价。已确定可以在直接DME合成路线中直接利用CO 2,而在甲醇/两步DME系统中将CO 2上游转化为CO是实现有效收率所必需的。通过H 2 O和CO 2或高温CO 2的高温共电解生产基于CO的合成气基于能耗和CO 2利用,电解与水煤气变换过程相结合被认为是最好的技术。


























 京公网安备 11010802027423号
京公网安备 11010802027423号