当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Basis of Analog Specificity in PKG I and II
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-08-22 00:00:00 , DOI: 10.1021/acschembio.7b00369 James C. Campbell 1, 2 , Philipp Henning 3 , Eugen Franz 3 , Banumathi Sankaran 4 , Friedrich W. Herberg 3 , Choel Kim 1, 2, 5
ACS Chemical Biology ( IF 4 ) Pub Date : 2017-08-22 00:00:00 , DOI: 10.1021/acschembio.7b00369 James C. Campbell 1, 2 , Philipp Henning 3 , Eugen Franz 3 , Banumathi Sankaran 4 , Friedrich W. Herberg 3 , Choel Kim 1, 2, 5
Affiliation
|
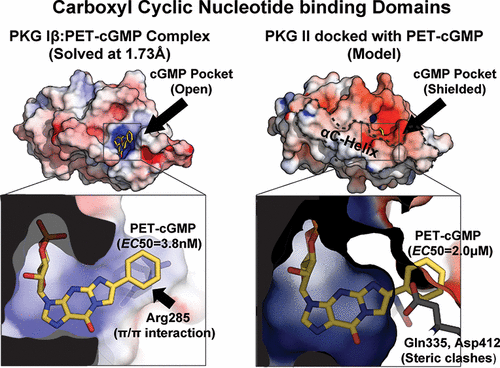
Cyclic GMP analogs, 8-Br, 8-pCPT, and PET-cGMP, have been widely used for characterizing cellular functions of cGMP-dependent protein kinase (PKG) I and II isotypes. However, interpreting results obtained using these analogs has been difficult due to their low isotype specificity. Additionally, each isotype has two binding sites with different cGMP affinities and analog selectivities, making understanding the molecular basis for isotype specificity of these compounds even more challenging. To determine isotype specificity of cGMP analogs and their structural basis, we generated the full-length regulatory domains of PKG I and II isotypes with each binding site disabled, determined their affinities for these analogs, and obtained cocrystal structures of both isotypes bound with cGMP analogs. Our affinity and activation measurements show that PET-cGMP is most selective for PKG I, whereas 8-pCPT-cGMP is most selective for PKG II. Our structures of cyclic nucleotide binding (CNB) domains reveal that the B site of PKG I is more open and forms a unique π/π interaction through Arg285 at β4 with the PET moiety, whereas the A site of PKG II has a larger β5/β6 pocket that can better accommodate the bulky 8-pCPT moiety. Our structural and functional results explain the selectivity of these analogs for each PKG isotype and provide a starting point for the rational design of isotype selective activators.
中文翻译:

PKG I和II中模拟特异性的结构基础
环状GMP类似物8-Br,8-pCPT和PET-cGMP已广泛用于表征cGMP依赖性蛋白激酶(PKG)I和II同型的细胞功能。然而,由于它们的同种型特异性低,使用这些类似物获得的解释结果非常困难。此外,每个同种型具有两个具有不同cGMP亲和力和类似物选择性的结合位点,这使得对这些化合物同种型特异性的分子基础进行了解更具挑战性。为了确定cGMP类似物的同型特异性及其结构基础,我们生成了PKG I和II同型的全长调控域,每个结合位点均被禁用,确定了它们对这些类似物的亲和力,并获得了与cGMP类似物结合的两个同型的共晶体结构。我们的亲和力和活化测试结果表明,PET-cGMP对PKG I最有选择性,而8-pCPT-cGMP对PKG II最有选择性。我们的环状核苷酸结合(CNB)域结构显示,PKG I的B位更开放,并且通过Arg285在β4与PET部分形成独特的π/π相互作用,而PKG II的A位具有更大的β5/可以更好地容纳庞大的8-pCPT部分的β6口袋。我们的结构和功能结果解释了这些类似物对每种PKG同种型的选择性,并为合理设计同种型选择性活化剂提供了起点。我们的环状核苷酸结合(CNB)域结构显示,PKG I的B位更开放,并且通过Arg285在β4与PET部分形成独特的π/π相互作用,而PKG II的A位具有更大的β5/可以更好地容纳庞大的8-pCPT部分的β6口袋。我们的结构和功能结果解释了这些类似物对每种PKG同种型的选择性,并为合理设计同种型选择性活化剂提供了起点。我们的环状核苷酸结合(CNB)域结构显示,PKG I的B位更开放,并且通过Arg285在β4与PET部分形成独特的π/π相互作用,而PKG II的A位具有更大的β5/可以更好地容纳庞大的8-pCPT部分的β6口袋。我们的结构和功能结果解释了这些类似物对每种PKG同种型的选择性,并为合理设计同种型选择性活化剂提供了起点。
更新日期:2017-08-23
中文翻译:

PKG I和II中模拟特异性的结构基础
环状GMP类似物8-Br,8-pCPT和PET-cGMP已广泛用于表征cGMP依赖性蛋白激酶(PKG)I和II同型的细胞功能。然而,由于它们的同种型特异性低,使用这些类似物获得的解释结果非常困难。此外,每个同种型具有两个具有不同cGMP亲和力和类似物选择性的结合位点,这使得对这些化合物同种型特异性的分子基础进行了解更具挑战性。为了确定cGMP类似物的同型特异性及其结构基础,我们生成了PKG I和II同型的全长调控域,每个结合位点均被禁用,确定了它们对这些类似物的亲和力,并获得了与cGMP类似物结合的两个同型的共晶体结构。我们的亲和力和活化测试结果表明,PET-cGMP对PKG I最有选择性,而8-pCPT-cGMP对PKG II最有选择性。我们的环状核苷酸结合(CNB)域结构显示,PKG I的B位更开放,并且通过Arg285在β4与PET部分形成独特的π/π相互作用,而PKG II的A位具有更大的β5/可以更好地容纳庞大的8-pCPT部分的β6口袋。我们的结构和功能结果解释了这些类似物对每种PKG同种型的选择性,并为合理设计同种型选择性活化剂提供了起点。我们的环状核苷酸结合(CNB)域结构显示,PKG I的B位更开放,并且通过Arg285在β4与PET部分形成独特的π/π相互作用,而PKG II的A位具有更大的β5/可以更好地容纳庞大的8-pCPT部分的β6口袋。我们的结构和功能结果解释了这些类似物对每种PKG同种型的选择性,并为合理设计同种型选择性活化剂提供了起点。我们的环状核苷酸结合(CNB)域结构显示,PKG I的B位更开放,并且通过Arg285在β4与PET部分形成独特的π/π相互作用,而PKG II的A位具有更大的β5/可以更好地容纳庞大的8-pCPT部分的β6口袋。我们的结构和功能结果解释了这些类似物对每种PKG同种型的选择性,并为合理设计同种型选择性活化剂提供了起点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号