当前位置:
X-MOL 学术
›
Mol. Biosyst.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into the RNA binding mechanism of human L1-ORF1p: a molecular dynamics study
Molecular BioSystems Pub Date : 2017-07-05 00:00:00 , DOI: 10.1039/c7mb00358g Muthukumaran Rajagopalan 1, 2, 3, 4 , Sangeetha Balasubramanian 1, 2, 3, 4 , Amutha Ramaswamy 1, 2, 3, 4
Molecular BioSystems Pub Date : 2017-07-05 00:00:00 , DOI: 10.1039/c7mb00358g Muthukumaran Rajagopalan 1, 2, 3, 4 , Sangeetha Balasubramanian 1, 2, 3, 4 , Amutha Ramaswamy 1, 2, 3, 4
Affiliation
|
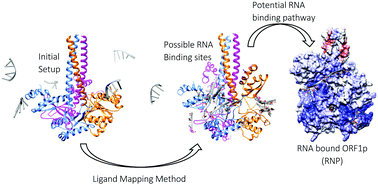
The recognition and binding of nucleic acids by ORF1p, an L1 retrotransposon protein, have not yet been clearly understood due to the lack of structural knowledge. The present study attempts to identify the probable single-stranded RNA binding pathway of trimeric ORF1p using computational methods like ligand mapping methodology combined with molecular dynamics simulations. Using the ligand mapping methodology, the possible RNA interacting sites on the surface of the trimeric ORF1p were identified. The crystal structure of the ORF1p timer and an RNA molecule of 29 nucleotide bases in length were used to generate the structure of the ORF1p complex based on information on predicted binding sites as well as the functional states of the CTD. The various complexes of ORF1p–RNA were generated using polyU, polyA and L1RNA sequences and were simulated for a period of 75 ns. The observed stable interaction pattern was used to propose the possible binding pathway. Based on the binding free energy for complex formation, both polyU and L1RNA complexes were identified as stable complexes, while the complex formed with polyA was the least stable one. Furthermore, the importance of the residues in the CC domain (Lys137 and Arg141), the RRM loop (Arg206, Arg210 and Arg211) and the CTD (Arg 261 and Arg262) of all three chains in stabilizing the wrapped RNA has been highlighted in this study. The presence of several electrostatic interactions including H-bond interactions increases the affinity towards RNA and hence plays a vital role in retaining the wrapped position of RNA around ORF1p. Altogether, this study presents one of the possible RNA binding pathways of ORF1p and clearly highlights the functional state of ORF1p visited during RNA binding.
中文翻译:

洞察人L1-ORF1p的RNA结合机制:分子动力学研究
由于缺乏结构知识,尚未清楚了解L1逆转座子蛋白ORF1p对核酸的识别和结合。本研究试图使用诸如配体作图法等结合分子动力学模拟的计算方法来确定三聚体ORF1p的可能的单链RNA结合途径。使用配体作图方法,确定了三聚体ORF1p表面上可能的RNA相互作用位点。基于关于预测结合位点的信息以及CTD的功能状态,使用ORF1p计时器的晶体结构和长度为29个核苷酸碱基的RNA分子来生成ORF1p复合物的结构。ORF1p–RNA的各种复合物是使用polyU生成的,polyA和L1RNA序列,并在75 ns的时间内进行了仿真。观察到的稳定相互作用模式用于提出可能的结合途径。基于复合物形成的结合自由能,polyU和L1RNA复合物均被鉴定为稳定的复合物,而与polyA形成的复合物是最不稳定的复合物。此外,本章还强调了这三个链的CC结构域(Lys137和Arg141),RRM环(Arg206,Arg210和Arg211)和CTD(Arg 261和Arg262)中的残基在稳定包装RNA中的重要性。学习。包括H键相互作用在内的几种静电相互作用的存在增加了对RNA的亲和力,因此在保持RNA围绕ORF1p的包裹位置方面起着至关重要的作用。共,
更新日期:2017-08-22
中文翻译:

洞察人L1-ORF1p的RNA结合机制:分子动力学研究
由于缺乏结构知识,尚未清楚了解L1逆转座子蛋白ORF1p对核酸的识别和结合。本研究试图使用诸如配体作图法等结合分子动力学模拟的计算方法来确定三聚体ORF1p的可能的单链RNA结合途径。使用配体作图方法,确定了三聚体ORF1p表面上可能的RNA相互作用位点。基于关于预测结合位点的信息以及CTD的功能状态,使用ORF1p计时器的晶体结构和长度为29个核苷酸碱基的RNA分子来生成ORF1p复合物的结构。ORF1p–RNA的各种复合物是使用polyU生成的,polyA和L1RNA序列,并在75 ns的时间内进行了仿真。观察到的稳定相互作用模式用于提出可能的结合途径。基于复合物形成的结合自由能,polyU和L1RNA复合物均被鉴定为稳定的复合物,而与polyA形成的复合物是最不稳定的复合物。此外,本章还强调了这三个链的CC结构域(Lys137和Arg141),RRM环(Arg206,Arg210和Arg211)和CTD(Arg 261和Arg262)中的残基在稳定包装RNA中的重要性。学习。包括H键相互作用在内的几种静电相互作用的存在增加了对RNA的亲和力,因此在保持RNA围绕ORF1p的包裹位置方面起着至关重要的作用。共,


























 京公网安备 11010802027423号
京公网安备 11010802027423号