Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2017-08-22 , DOI: 10.1016/j.bmc.2017.08.030 Sateesh Kumar Arepalli , Byeongwoo Park , Kiho Lee , Hyunji Jo , Kyu-Yeon Jun , Youngjoo Kwon , Jong-Soon Kang , Jae-Kyung Jung , Heesoon Lee
|
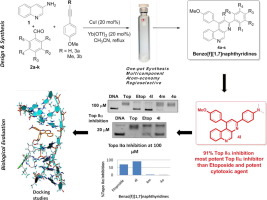
A novel series of twenty 1,3-diphenylbenzo[f][1,7]benzonaphthyrdine derivatives were designed and synthesized through intermolecular imino Diels-Alder reaction. Their in vitro cytotoxic activities were evaluated against six human cancer cell lines (NCIH23, HCT15, NUGC-3, ACHN, PC-3, and MDA-MB-231). Majority of synthesized compounds exhibited significant cytotoxic activities against all tested human cancer cell lines. Among them 4l, 4m, and 4o derivatives exhibited most promising cytotoxic activities. Furthermore these compounds were evaluated against human Topoisomerase IIα inhibition. Interestingly, the compound 4l exhibited 1.3 and 1.2 times more potent human Topoisomerase IIα inhibition than the reference drug etoposide in both 100 µM and 20 µM concentrations respectively. Molecular docking studies for the compound 4l have also been executed by Sybyl X-2.1 in which it reveals the binding site of the compound 4l with topo IIα DNA cleavage site where etoposide was situated. The benzo[f][1,7]naphthyridine ring was stacked between the DNA bases of the cleavage site.
中文翻译:

1,3-二苯基苯并[ f ] [1,7]萘啶的设计,合成及生物学评价
通过分子间亚氨基Diels-Alder反应设计合成了一系列新的二十种1,3-二苯基苯并[ f ] [1,7]苯并萘啶衍生物。评估了它们对六种人类癌细胞系(NCIH23,HCT15,NUGC-3,ACHN,PC-3和MDA-MB-231)的体外细胞毒性活性。大多数合成的化合物对所有测试的人类癌细胞系均表现出显着的细胞毒性活性。其中4l,4m和4o衍生物表现出最有希望的细胞毒活性。此外,评估了这些化合物对人拓扑异构酶IIα的抑制作用。有趣的是,化合物4l在浓度分别为100 µM和20 µM时,它们对人拓扑异构酶IIα的抑制作用分别是参考药物依托泊苷的1.3和1.2倍。该化合物的分子对接研究4 l具备也被SYBYL X-2.1,其中它揭示了化合物的结合位点执行4升与TOPOIIαDNA切割位点,其中依托泊苷位于。苯并[ f ] [1,7]萘啶环堆叠在切割位点的DNA碱基之间。



























 京公网安备 11010802027423号
京公网安备 11010802027423号