Nature Chemical Biology ( IF 14.8 ) Pub Date : 2017-08-18 , DOI: 10.1038/nchembio.2431 J Robert Lane , Lauren T May , Robert G Parton , Patrick M Sexton , Arthur Christopoulos
|
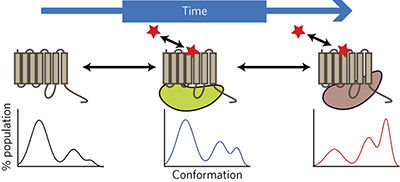
G-protein-coupled receptors (GPCRs) are one of the most tractable classes of drug targets. These dynamic proteins can adopt multiple active states that are linked to distinct functional outcomes. Such states can be differentially stabilized by ligands interacting with the endogenous agonist-binding orthosteric site and/or by ligands acting via spatially distinct allosteric sites, leading to the phenomena of 'biased agonism' or 'biased modulation'. These paradigms are having a major impact on modern drug discovery, but it is becoming increasingly apparent that 'kinetic context', at the level of both ligand–receptor and receptor—signal pathway kinetics, can have a profound impact on the observation and quantification of these phenomena. The concept of kinetic context thus represents an important new consideration that should be routinely incorporated into contemporary chemical biology and drug discovery studies of GPCR bias and allostery.
中文翻译:

GPCR变构和偏向激动的动力学观点
G蛋白偶联受体(GPCR)是药物靶标中最易处理的一类。这些动态蛋白质可以采用与不同功能结果相关的多个活性状态。这种状态可以通过与内源性激动剂结合的正构位点相互作用的配体和/或通过在空间上不同的别构位点起作用的配体而被不同地稳定,从而导致“偏向激动作用”或“偏向调节”现象。这些范例对现代药物发现产生了重大影响,但是越来越明显的是,在配体-受体和受体-信号途径动力学水平上的“动力学背景”可以对药物的观察和定量产生深远的影响。这些现象。

























 京公网安备 11010802027423号
京公网安备 11010802027423号