当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catabolism of the Cholesterol Side Chain in Mycobacterium tuberculosis Is Controlled by a Redox-Sensitive Thiol Switch
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1021/acsinfecdis.7b00072 Rui Lu 1 , Christin M. Schaefer 2 , Natasha M. Nesbitt 1 , Jochen Kuper 2 , Caroline Kisker 2 , Nicole S. Sampson 1, 3
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1021/acsinfecdis.7b00072 Rui Lu 1 , Christin M. Schaefer 2 , Natasha M. Nesbitt 1 , Jochen Kuper 2 , Caroline Kisker 2 , Nicole S. Sampson 1, 3
Affiliation
|
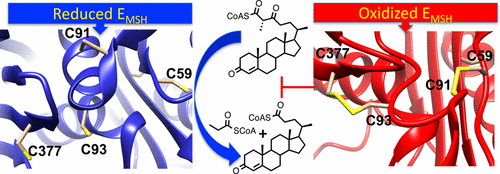
Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), is a highly successful human pathogen and has infected approximately one-third of the world’s population. Multiple drug resistant (MDR) and extensively drug resistant (XDR) TB strains and coinfection with HIV have increased the challenges of successfully treating this disease pandemic. The metabolism of host cholesterol by Mtb is an important factor for both its virulence and pathogenesis. In Mtb, the cholesterol side chain is degraded through multiple cycles of β-oxidation and FadA5 (Rv3546) catalyzes side chain thiolysis in the first two cycles. Moreover, FadA5 is important during the chronic stage of infection in a mouse model of Mtb infection. Here, we report the redox control of FadA5 catalytic activity that results from reversible disulfide bond formation between Cys59-Cys91 and Cys93-Cys377. Cys93 is the thiolytic nucleophile, and Cys377 is the general acid catalyst for cleavage of the β-keto-acyl-CoA substrate. The disulfide bond formed between the two catalytic residues Cys93 and Cys377 blocks catalysis. The formation of the disulfide bonds is accompanied by a large domain swap at the FadA5 dimer interface that serves to bring Cys93 and Cys377 in close proximity for disulfide bond formation. The catalytic activity of FadA5 has a midpoint potential of −220 mV, which is close to the Mtb mycothiol potential in the activated macrophage. The redox profile of FadA5 suggests that FadA5 is fully active when Mtb resides in the unactivated macrophage to maximize flux into cholesterol catabolism. Upon activation of the macrophage, the oxidative shift in the mycothiol potential will decrease the thiolytic activity by 50%. Thus, the FadA5 midpoint potential is poised to rapidly restrict cholesterol side chain degradation in response to oxidative stress from the host macrophage environment.
中文翻译:

结核分枝杆菌中胆固醇侧链的分解代谢受氧化还原敏感硫醇开关控制。
结核分枝杆菌(TB)的病原体结核分枝杆菌(Mtb)是一种非常成功的人类病原体,已感染了全球约三分之一的人口。多重耐药(MDR)和广泛耐药(XDR)TB菌株以及HIV共感染增加了成功治疗这种疾病大流行的挑战。Mtb对宿主胆固醇的代谢是其毒性和发病机理的重要因素。在Mtb中,胆固醇的侧链通过多个β-氧化循环而降解,而FadA5(Rv3546)在前两个循环中催化侧链硫解。此外,FadA5在Mtb小鼠模型的慢性感染阶段很重要感染。在这里,我们报告FadA5催化活性的氧化还原控制,这是由于Cys59-Cys91和Cys93-Cys377之间可逆的二硫键形成所致。Cys93是硫解亲核试剂,Cys377是裂解β-酮-酰基-CoA底物的通用酸催化剂。在两个催化残基Cys93和Cys377之间形成的二硫键会阻止催化作用。二硫键的形成在FadA5二聚体界面处伴随着一个大的结构域交换,从而使Cys93和Cys377紧密靠近以形成二硫键。FadA5的催化活性的中点电位为-220 mV,接近活化的巨噬细胞中的Mtb麦硫酚电位。FadA5的氧化还原谱表明当Mtb时FadA5完全活跃驻留在未活化的巨噬细胞中,以最大程度地提高胆固醇分解代谢的通量。巨噬细胞激活后,霉菌硫醇电位的氧化位移将使硫解活性降低50%。因此,FadA5中点电位有望响应来自宿主巨噬细胞环境的氧化应激而迅速限制胆固醇侧链降解。
更新日期:2017-08-08
中文翻译:

结核分枝杆菌中胆固醇侧链的分解代谢受氧化还原敏感硫醇开关控制。
结核分枝杆菌(TB)的病原体结核分枝杆菌(Mtb)是一种非常成功的人类病原体,已感染了全球约三分之一的人口。多重耐药(MDR)和广泛耐药(XDR)TB菌株以及HIV共感染增加了成功治疗这种疾病大流行的挑战。Mtb对宿主胆固醇的代谢是其毒性和发病机理的重要因素。在Mtb中,胆固醇的侧链通过多个β-氧化循环而降解,而FadA5(Rv3546)在前两个循环中催化侧链硫解。此外,FadA5在Mtb小鼠模型的慢性感染阶段很重要感染。在这里,我们报告FadA5催化活性的氧化还原控制,这是由于Cys59-Cys91和Cys93-Cys377之间可逆的二硫键形成所致。Cys93是硫解亲核试剂,Cys377是裂解β-酮-酰基-CoA底物的通用酸催化剂。在两个催化残基Cys93和Cys377之间形成的二硫键会阻止催化作用。二硫键的形成在FadA5二聚体界面处伴随着一个大的结构域交换,从而使Cys93和Cys377紧密靠近以形成二硫键。FadA5的催化活性的中点电位为-220 mV,接近活化的巨噬细胞中的Mtb麦硫酚电位。FadA5的氧化还原谱表明当Mtb时FadA5完全活跃驻留在未活化的巨噬细胞中,以最大程度地提高胆固醇分解代谢的通量。巨噬细胞激活后,霉菌硫醇电位的氧化位移将使硫解活性降低50%。因此,FadA5中点电位有望响应来自宿主巨噬细胞环境的氧化应激而迅速限制胆固醇侧链降解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号