Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2017-08-12 , DOI: 10.1016/j.apcata.2017.08.012 D.S. Paz , S. Damyanova , L.R. Borges , J.B.O Santos , J.M.C. Bueno
|
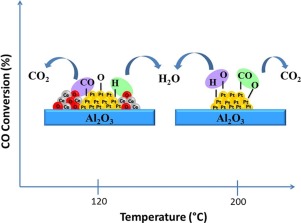
A series of non- and modified with niobia and ceria alumina-supported Pt catalysts with different Pt content (0.3–2 wt%) were prepared by wetness impregnation method and characterized by N2 adsorption-desorption isotherms, XRD, TPR, DRIFT, in situ EXAFS and ΔXANES. The effect of the content of Pt and promoter on the change of catalyst electronic structure and behaviors in the reaction of preferential oxidation of CO in the presence of H2 was studied. A strong interaction of Pt with CO and O2 in atmospheres of H2/CO and H2/CO/O2 mixtures was detected by ΔXANES. Different steps of activation of reagents molecules were observed. The increase of activity with increasing the Pt content was related to the presence of Pt nanoparticles with high electron density. While, the highest metal dispersion of the low metal-loaded Pt catalyst was responsible for its high CO oxidation selectivity. Addition of ceria to Pt catalysts caused significantly increase of activity and CO oxidation selectivity at lower reaction temperatures. Niobia addition did not improve the catalysts behaviors. The promotion effect of ceria was related to the oxygen mobility at PtO
Ce interface. The ceria-promoted Pt catalysts with the highest ceria loading of 12 wt% exhibited the highest activity and CO oxidation selectivity, but at higher reaction temperatures of about 110°–130 °C it was begin slowly to decrease. This was attributed to going on the reverse of WGS reaction and high O2 consumption to H2 oxidation during the PROX-CO reaction on ceria alumina-supported Pt catalysts at high temperatures.
中文翻译:

识别Pt表面吸附的活性中间体,并通过氧化还原CeO 2促进H 2中CO优先氧化中的活性
通过湿法浸渍法制备了一系列不同的,由铌和二氧化铈负载的氧化铈和氧化铈氧化铝负载的Pt催化剂(0.3–2 wt%),并通过N 2吸附-脱附等温线,XRD,TPR,DRIFT表征。原位EXAFS和ΔXANES。研究了Pt和助催化剂的含量对H 2存在下CO优先氧化反应中催化剂电子结构和行为的影响。H 2 / CO和H 2 / CO / O 2气氛中Pt与CO和O 2的强相互作用通过ΔXANES检测混合物。观察到了试剂分子活化的不同步骤。随着Pt含量的增加,活性的增加与具有高电子密度的Pt纳米颗粒的存在有关。同时,低金属负载的Pt催化剂的最高金属分散度是其高CO氧化选择性的原因。在较低的反应温度下,将二氧化铈添加到Pt催化剂中可显着提高活性和CO氧化选择性。纳米比亚的添加并没有改善催化剂的行为。二氧化铈的促进作用与Pt O处的氧迁移率有关
Ce接口。二氧化铈含量最高的氧化铈负载的Pt催化剂具有12 wt%的活性和最高的CO氧化选择性,但在约110°–130°C的较高反应温度下,它开始缓慢降低。这归因于在高温下在二氧化铈氧化铝负载的Pt催化剂上的PROX-CO反应期间,WGS反应和高O 2消耗与H 2氧化相反。



























 京公网安备 11010802027423号
京公网安备 11010802027423号