当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and evaluation of oxindoles as promising inhibitors of the immunosuppressive enzyme indoleamine 2,3-dioxygenase 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-06-20 00:00:00 , DOI: 10.1039/c7md00226b Saurav Paul 1, 2, 3 , Ashalata Roy 1, 2, 3 , Suman Jyoti Deka 2, 3, 4 , Subhankar Panda 1, 2, 3 , Gopal Narayan Srivastava 1, 2, 3 , Vishal Trivedi 2, 3, 4 , Debasis Manna 1, 2, 3
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-06-20 00:00:00 , DOI: 10.1039/c7md00226b Saurav Paul 1, 2, 3 , Ashalata Roy 1, 2, 3 , Suman Jyoti Deka 2, 3, 4 , Subhankar Panda 1, 2, 3 , Gopal Narayan Srivastava 1, 2, 3 , Vishal Trivedi 2, 3, 4 , Debasis Manna 1, 2, 3
Affiliation
|
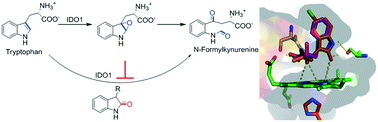
Indoleamine 2,3-dioxygenase 1 (IDO1) is considered as an important therapeutic target for the treatment of cancer, chronic infections and other diseases that are associated with immune suppression. Recent developments in understanding the catalytic mechanism of the IDO1 enzyme revealed that conversion of L-tryptophan (L-Trp) to N-formylkynurenine proceeded through an epoxide intermediate state. Accordingly, we synthesized a series of 3-substituted oxindoles from L-Trp, tryptamine and isatin. Compounds with C3-substituted oxindole moieties showed moderate inhibitory activity against the purified human IDO1 enzyme. Their optimization led to the identification of potent compounds, 6, 22, 23 and 25 (IC50 = 0.19 to 0.62 μM), which are competitive inhibitors of IDO1 with respect to L-Trp. These potent compounds also showed IDO1 inhibition potencies in the low-micromolar range (IC50 = 0.33–0.49 μM) in MDA-MB-231 cells. The cytotoxicity of these potent compounds was trivial in different model cancer (MDA-MB-231, A549 and HeLa) cells and macrophage (J774A.1) cells. Stronger selectivity for the IDO1 enzyme (124 to 210-fold) over the tryptophan 2,3-dioxygenase (TDO) enzyme was also observed for these compounds. These results suggest that the oxindole moiety of the compounds could mimic the epoxide intermediate state of L-Trp. Therefore, the structural simplicity and low-micromolar inhibition potencies of these 3-substituted oxindoles make them quite attractive for further investigation of IDO1 function and immunotherapeutic applications.
中文翻译:

羟吲哚作为免疫抑制酶吲哚胺2,3-双加氧酶1的有前途的抑制剂的合成与评估
吲哚胺2,3-二加氧酶1(IDO1)被认为是治疗癌症,慢性感染和其他与免疫抑制有关的疾病的重要治疗靶标。在理解IDO1酶的催化机理方面的最新进展表明,L-色氨酸(L- Trp )向N-甲酰基kynurenine的转化通过环氧化物中间态进行。因此,我们从L -Trp,色胺和靛红合成了一系列3-取代的羟吲哚。具有C3取代的羟吲哚基团的化合物对纯化的人IDO1酶表现出中等的抑制活性。他们的优化导致了鉴定有效的化合物的,6,22,23和25(IC 50 = 0.19至0.62μM),它们是IDO1相对于L -Trp的竞争性抑制剂。这些有效的化合物还在MDA-MB-231细胞中的低微摩尔范围(IC 50 = 0.33–0.49μM)中显示出IDO1抑制能力。这些有效化合物在不同模型的癌细胞(MDA-MB-231,A549和HeLa)和巨噬细胞(J774A.1)中的细胞毒性微不足道。对于这些化合物,还观察到IDO1酶比色氨酸2,3-二加氧酶(TDO)酶具有更高的选择性(124到210倍)。这些结果表明,化合物的羟吲哚部分可以模拟L的环氧化物中间状态。-Trp。因此,这些3-取代的羟吲哚的结构简单性和低微摩尔抑制潜能使其对IDO1功能和免疫治疗应用的进一步研究具有很大的吸引力。
更新日期:2017-08-16
中文翻译:

羟吲哚作为免疫抑制酶吲哚胺2,3-双加氧酶1的有前途的抑制剂的合成与评估
吲哚胺2,3-二加氧酶1(IDO1)被认为是治疗癌症,慢性感染和其他与免疫抑制有关的疾病的重要治疗靶标。在理解IDO1酶的催化机理方面的最新进展表明,L-色氨酸(L- Trp )向N-甲酰基kynurenine的转化通过环氧化物中间态进行。因此,我们从L -Trp,色胺和靛红合成了一系列3-取代的羟吲哚。具有C3取代的羟吲哚基团的化合物对纯化的人IDO1酶表现出中等的抑制活性。他们的优化导致了鉴定有效的化合物的,6,22,23和25(IC 50 = 0.19至0.62μM),它们是IDO1相对于L -Trp的竞争性抑制剂。这些有效的化合物还在MDA-MB-231细胞中的低微摩尔范围(IC 50 = 0.33–0.49μM)中显示出IDO1抑制能力。这些有效化合物在不同模型的癌细胞(MDA-MB-231,A549和HeLa)和巨噬细胞(J774A.1)中的细胞毒性微不足道。对于这些化合物,还观察到IDO1酶比色氨酸2,3-二加氧酶(TDO)酶具有更高的选择性(124到210倍)。这些结果表明,化合物的羟吲哚部分可以模拟L的环氧化物中间状态。-Trp。因此,这些3-取代的羟吲哚的结构简单性和低微摩尔抑制潜能使其对IDO1功能和免疫治疗应用的进一步研究具有很大的吸引力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号