PLOS Medicine ( IF 15.8 ) Pub Date : 2017-08-15 , DOI: 10.1371/journal.pmed.1002369 Harsha Shanthanna , Ian Gilron , Manikandan Rajarathinam , Rizq AlAmri , Sriganesh Kamath , Lehana Thabane , Philip J. Devereaux , Mohit Bhandari
|
Background and objective
Chronic Low Back Pain (CLBP) is very common, with a lifetime prevalence between 51% and 80%. In majority, it is nonspecific in nature and multifactorial in etiology. Pregabalin (PG) and Gabapentin (GB) are gabapentinoids that have demonstrated benefit in neuropathic pain conditions. Despite no clear rationale, they are increasingly used for nonspecific CLBP. They necessitate prolonged use and are associated with adverse effects and increased cost. Recent guidelines from the National Health Service (NHS), England, expressed concerns on their off-label use, in addition to the risk of misuse. We aimed to assess the effectiveness and safety of gabapentinoids in adult CLBP patients.
Methods
Electronic databases of MEDLINE, EMBASE, and Cochrane were searched from their inception until December 20th, 2016. We included randomized control trials reporting the use of gabapentinoids for the treatment of CLBP of >3 months duration, in adult patients. Study selection and data extraction was performed independently by paired reviewers. Outcomes were guided by Initiative on Methods, Measurement and Pain Assessment in Clinical Trials guidelines, with pain relief and safety as the primary outcomes. Meta-analyses were performed for outcomes reported in 3 or more studies. Outcomes were reported as mean differences (MDs) or risk ratios (RRs) with their corresponding 95% confidence intervals (CIs), and I2 in percentage representing the percentage variability in effect estimates that could be explained by heterogeneity. GRADE (Grading of Recommendations Assessment, Development, and Evaluation) was used to assess the quality of evidence.
Results
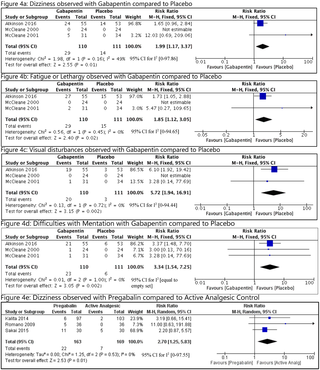
Out of 1,385 citations, eight studies were included. Based on the interventions and comparators, studies were analyzed in 3 different groups. GB compared with placebo (3 studies, n = 185) showed minimal improvement of pain (MD = 0.22 units, 95% CI [−0.5 to 0.07] I2 = 0%; GRADE: very low). Three studies compared PG with other types of analgesic medication (n = 332) and showed greater improvement in the other analgesic group (MD = 0.42 units, 95% CI [0.20 to 0.64] I2 = 0; GRADE: very low). Studies using PG as an adjuvant (n = 423) were not pooled due to heterogeneity, but the largest of them showed no benefit of adding PG to tapentadol. There were no deaths or hospitalizations reported. Compared with placebo, the following adverse events were more commonly reported with GB: dizziness-(RR = 1.99, 95% CI [1.17 to 3.37], I2 = 49); fatigue (RR = 1.85, 95% CI [1.12 to 3.05], I2 = 0); difficulties with mentation (RR = 3.34, 95% CI [1.54 to 7.25], I2 = 0); and visual disturbances (RR = 5.72, 95% CI [1.94 to 16.91], I2 = 0). The number needed to harm with 95% CI for dizziness, fatigue, difficulties with mentation, and visual disturbances were 7 (4 to 30), 8 (4 to 44), 6 (4 to 15), and 6 (4 to 13) respectively. The GRADE evidence quality was noted to be very low for dizziness and fatigue, low for difficulties with mentation, and moderate for visual disturbances. Functional and emotional improvements were reported by few studies and showed no significant improvements.
Conclusions and relevance
Existing evidence on the use of gabapentinoids in CLBP is limited and demonstrates significant risk of adverse effects without any demonstrated benefit. Given the lack of efficacy, risks, and costs associated, the use of gabapentinoids for CLBP merits caution. There is need for large high-quality trials to more definitively inform this issue.
Trial registration
PROSPERO CRD42016034040
中文翻译:

加巴喷丁类药物在慢性下腰痛中的益处和安全性:随机对照试验的系统评价和荟萃分析
背景和目标
慢性腰背痛(CLBP)非常常见,终生患病率在51%至80%之间。大多数情况下,它在性质上是非特异性的,在病因学上是多因素的。普瑞巴林(PG)和加巴喷丁(GB)是加巴喷丁类药物,已证明对神经性疼痛症状有益。尽管没有明确的理由,但它们越来越多地用于非特异性CLBP。它们需要长时间使用,并伴有不良影响和成本增加。英格兰国家卫生局(NHS)的最新指南表示,除误用的风险外,还对标签外使用表示担忧。我们旨在评估加巴喷丁类药物在成人CLBP患者中的有效性和安全性。
方法
从MEDLINE,EMBASE和Cochrane的电子数据库开始搜索,直到2016年12月20日。我们纳入了随机对照试验,这些试验报告了在成人患者中使用加巴喷丁类药物治疗持续时间超过3个月的CLBP。研究选择和数据提取由配对的审阅者独立进行。结果以临床试验指南中的方法,测量和疼痛评估倡议为指导,以缓解疼痛和安全性为主要结果。对3项或3项以上研究报告的结局进行Meta分析。结果以平均差异(MDs)或风险比(RRs)以及相应的95%置信区间(CIs)和I 2来报告。以百分比表示可以通过异质性解释的效果估计中的百分比可变性。GRADE(建议评估,制定和评估的等级)用于评估证据的质量。
结果
在1,385篇文献中,包括八项研究。基于干预措施和比较者,对3个不同的组进行了研究分析。GB与安慰剂相比(3个研究,n = 185)显示疼痛改善最小(MD = 0.22单位,95%CI [-0.5至0.07] I 2 = 0%; GRADE:非常低)。三项研究比较了PG与其他类型的镇痛药(n = 332),并显示了其他镇痛药组的改善更大(MD = 0.42单位,95%CI [0.20至0.64] I 2 = 0; GRADE:非常低)。使用PG作为佐剂的研究(n= 423)由于异质性而没有合并,但其中最大的合并显示没有将PG添加到他喷他多中的益处。没有死亡或住院的报道。与安慰剂相比,GB常见以下不良事件:头晕-(RR = 1.99,95%CI [1.17至3.37],I 2 = 49);疲劳(RR = 1.85,95%CI [1.12至3.05],I 2 = 0);精神障碍(RR = 3.34,95%CI [1.54 to 7.25],I 2 = 0);和视觉障碍(RR = 5.72,95%CI [1.94至16.91],I 2= 0)。因头晕,疲劳,精神错乱和视觉障碍而需要对95%CI进行伤害的数字是7(4至30),8(4至44),6(4至15)和6(4至13)分别。对于头晕和疲劳,GRADE证据质量非常低,对精神障碍的质量很低,对于视觉障碍的质量也很适中。鲜有研究报告功能和情绪改善,但无明显改善。
结论与相关性
关于在CLBP中使用加巴喷丁类药物的现有证据有限,并且显示出不良反应的风险很高,而没有任何已证实的益处。鉴于缺乏功效,风险和相关成本,使用加巴喷丁类药物治疗CLBP值得谨慎。需要进行大规模的高质量试验才能更明确地告知这一问题。



























 京公网安备 11010802027423号
京公网安备 11010802027423号