当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chelation-Controlled Additions to Chiral α- and β-Silyloxy, α-Halo, and β-Vinyl Carbonyl Compounds
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1021/acs.accounts.7b00319 Xinyuan Fan 1 , Patrick J. Walsh 1, 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1021/acs.accounts.7b00319 Xinyuan Fan 1 , Patrick J. Walsh 1, 2
Affiliation
|
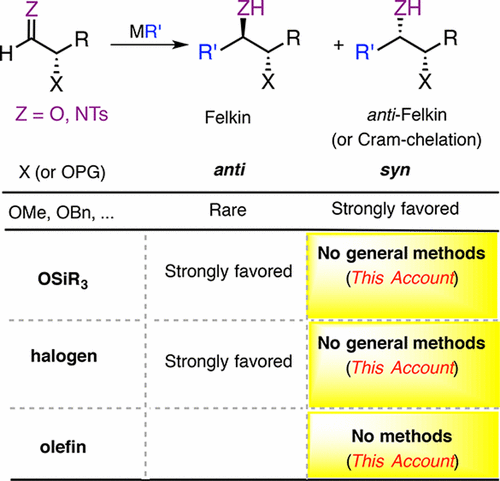
The science and art of preventing and managing disease and prolonging life is dependent on advances in medicine, biology, and biochemistry. Many of these advances will involve interactions of small molecules with biological entities. As such, they will rely on the efficient synthesis of active compounds with very high stereochemical purity. Although enantioselective reactions are important in this regard, most stereocenters in complex molecule synthesis are installed in diastereoselective reactions. Perhaps the most well-known diastereoselective C–C bond-forming reaction is the addition of nucleophiles to carbonyl groups with α- or β-stereogenic centers. Diastereoselective additions of organometallic reagents to protected chiral α- and β-hydroxy aldehydes and ketones are described by either Cram chelation or Felkin–Anh models, which are protecting group (PG)-dependent. Small PGs (X = OMOM, OBn, etc.) favor Cram chelation, wherein both the carbonyl group and the O-PG bind to the Lewis acidic metal, providing syn diol motifs. In contrast, silyl PGs, with the OSiR3 moiety being both bulky and weakly coordinating, provide anti diols (Felkin addition). It is well-known that exceptions to this paradigm are scarce. Therefore, the choice of PG is based on the desired stereochemical outcome in the addition step and is often inappropriate for the global protection strategy. Thus, it is critical to develop general methods for chelation-controlled additions of organometallics to chiral silyloxy aldehydes and ketones. Once the challenge of developing chelation-controlled additions to silyloxy carbonyl compounds can be met, the next question is what other pendant functional groups can chelate?
中文翻译:

手性α-和β-甲硅烷氧基,α-卤代和β-乙烯基羰基化合物的螯合控制加成
预防和控制疾病并延长寿命的科学和技术取决于医学,生物学和生物化学的进步。这些进步中有许多将涉及小分子与生物实体的相互作用。因此,它们将依赖于具有非常高的立体化学纯度的活性化合物的有效合成。尽管对映选择性反应在这方面很重要,但复杂分子合成中的大多数立体中心都安装在非对映选择性反应中。也许最著名的非对映选择性C–C键形成反应是将亲核试剂添加到具有α-或β-立体异构中心的羰基上。非对映选择性添加有机金属试剂Cram螯合或Felkin-Anh模型描述了受保护的手性α-和β-羟基醛和酮的存在,它们是依赖于保护基团(PG)的。小型PG(X = OMOM,OBn等)有利于Cram螯合,其中羰基和O-PG均与Lewis酸性金属结合,从而提供合成二醇基序。相比之下,OSiR 3部分既庞大又不易配位的甲硅烷基PG可提供抗二醇(Felkin加法)。众所周知,这种范例的例外很少。因此,PG的选择基于添加步骤中所需的立体化学结果,并且通常不适用于整体保护策略。因此,开发通用的方法是控制有机金属向手性甲硅烷氧基醛和酮的螯合控制加成。一旦解决了开发对甲硅烷氧基羰基化合物进行螯合控制的加成的挑战,下一个问题便是其他哪些侧基官能团可以螯合?
更新日期:2017-08-15
中文翻译:

手性α-和β-甲硅烷氧基,α-卤代和β-乙烯基羰基化合物的螯合控制加成
预防和控制疾病并延长寿命的科学和技术取决于医学,生物学和生物化学的进步。这些进步中有许多将涉及小分子与生物实体的相互作用。因此,它们将依赖于具有非常高的立体化学纯度的活性化合物的有效合成。尽管对映选择性反应在这方面很重要,但复杂分子合成中的大多数立体中心都安装在非对映选择性反应中。也许最著名的非对映选择性C–C键形成反应是将亲核试剂添加到具有α-或β-立体异构中心的羰基上。非对映选择性添加有机金属试剂Cram螯合或Felkin-Anh模型描述了受保护的手性α-和β-羟基醛和酮的存在,它们是依赖于保护基团(PG)的。小型PG(X = OMOM,OBn等)有利于Cram螯合,其中羰基和O-PG均与Lewis酸性金属结合,从而提供合成二醇基序。相比之下,OSiR 3部分既庞大又不易配位的甲硅烷基PG可提供抗二醇(Felkin加法)。众所周知,这种范例的例外很少。因此,PG的选择基于添加步骤中所需的立体化学结果,并且通常不适用于整体保护策略。因此,开发通用的方法是控制有机金属向手性甲硅烷氧基醛和酮的螯合控制加成。一旦解决了开发对甲硅烷氧基羰基化合物进行螯合控制的加成的挑战,下一个问题便是其他哪些侧基官能团可以螯合?



























 京公网安备 11010802027423号
京公网安备 11010802027423号