当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bioinspired Indole Prenylation Reactions in Water
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-08-13 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00464 Satomi Tanaka 1 , Shinya Shiomi 1 , Hayato Ishikawa 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-08-13 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00464 Satomi Tanaka 1 , Shinya Shiomi 1 , Hayato Ishikawa 1
Affiliation
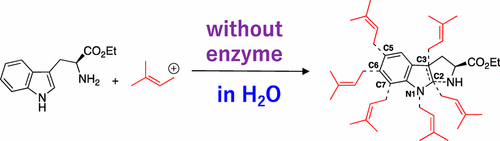
|
Isoprene units derived from dimethylallyl diphosphate (DMAPP) are an important motif in many natural products including terpenoids, carotenoids, steroids, and natural rubber. Understanding the chemical characteristics of DMAPP is an important topic in natural products chemistry, organic chemistry, and biochemistry. We have developed a direct bioinspired indole prenylation reaction using DMAPP or its equivalents as the electrophile in homogeneous aqueous acidic media in the absence of enzyme to provide prenylated indole products. After establishing the bioinspired indole prenylation reaction, this was then used to achieve the synthesis of a series of natural products, namely, N-prenylcyclo-l-tryptophyl-l-proline, tryprostatins, rhinocladins, and terezine D.
中文翻译:

生物启发的水中吲哚异戊烯化反应
衍生自二甲基二烯丙基二磷酸酯(DMAPP)的异戊二烯单元是许多天然产物(包括萜类,类胡萝卜素,类固醇和天然橡胶)中的重要基序。了解DMAPP的化学特性是天然产物化学,有机化学和生物化学中的重要主题。我们已经开发出一种直接的生物启发型吲哚异戊二烯化反应,使用DMAPP或其等同物作为亲电试剂,在均质水性酸性介质中,在不存在酶的情况下提供了异戊二烯化的吲哚产物。建立所述仿生吲哚异戊烯化反应后,然后将其用于实现一系列天然产物,即,的合成Ñ -prenylcyclo-升-tryptophyl-升-脯氨酸,tryprostatins,rhinocladins和terezine D.
更新日期:2017-08-13
中文翻译:

生物启发的水中吲哚异戊烯化反应
衍生自二甲基二烯丙基二磷酸酯(DMAPP)的异戊二烯单元是许多天然产物(包括萜类,类胡萝卜素,类固醇和天然橡胶)中的重要基序。了解DMAPP的化学特性是天然产物化学,有机化学和生物化学中的重要主题。我们已经开发出一种直接的生物启发型吲哚异戊二烯化反应,使用DMAPP或其等同物作为亲电试剂,在均质水性酸性介质中,在不存在酶的情况下提供了异戊二烯化的吲哚产物。建立所述仿生吲哚异戊烯化反应后,然后将其用于实现一系列天然产物,即,的合成Ñ -prenylcyclo-升-tryptophyl-升-脯氨酸,tryprostatins,rhinocladins和terezine D.

























 京公网安备 11010802027423号
京公网安备 11010802027423号