当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deciphering the Fluorine Code—The Many Hats Fluorine Wears in a Protein Environment
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-08-12 00:00:00 , DOI: 10.1021/acs.accounts.7b00226 Allison Ann Berger 1 , Jan-Stefan Völler 2 , Nediljko Budisa 2 , Beate Koksch 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2017-08-12 00:00:00 , DOI: 10.1021/acs.accounts.7b00226 Allison Ann Berger 1 , Jan-Stefan Völler 2 , Nediljko Budisa 2 , Beate Koksch 1
Affiliation
|
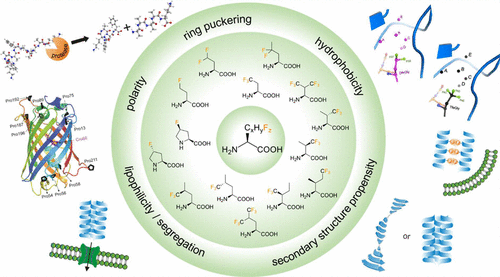
Deciphering the fluorine code is how we describe not only the focus of this Account, but also the systematic approach to studying the impact of fluorine’s incorporation on the properties of peptides and proteins used by our groups and others. The introduction of fluorine has been shown to impart favorable, but seldom predictable, properties to peptides and proteins, but up until about two decades ago the outcomes of fluorine modification of peptides and proteins were largely left to chance. Driven by the motivation to extend the application of the unique properties of the element fluorine from medicinal and agro chemistry to peptide and protein engineering we have established extensive research programs that enable the systematic investigation of effects that accompany the introduction of fluorine into this class of biopolymers. The introduction of fluorine into amino acids offers a universe of options for modifications with regard to number and position of fluorine substituents in the amino acid side chain. Moreover, it is important to emphasize that the consequences of incorporating the C–F bond into a biopolymer can be attributed to two distinct yet related phenomena: (i) the fluorine substituent can directly engage in intermolecular interactions with its environment and/or (ii) the other functional groups present in the molecule can be influenced by the electron withdrawing nature of this element (intramolecular) and in turn interact differently with their immediate environment (intermolecular). Based on our studies, we have shown that a change in number and/or position of as subtle as one single fluorine substituent has the power to considerably modify key properties of amino acids such as hydrophobicity, polarity, and secondary structure propensity. These properties are crucial factors in peptide and protein engineering, and thus, fluorinated amino acids can be applied to fine-tune properties such as protein folding, proteolytic stability, and protein–protein interactions provided we understand and become able to predict the outcome of a fluorine substitution in this context. With this Account, we attempt to analyze information we gained from our recent projects on how the nature of the fluorine atom and C–F bond influence four key properties of peptides and proteins: peptide folding, protein–protein interactions, ribosomal translation, and protease stability. These results impressively show why the introduction of fluorine creates a new class of amino acids with a repertoire of functionalities that is unique to the world of proteins and in some cases orthogonal to the set of canonical and natural amino acids. Our concluding statements aim to offer a few conserved design principles that have emerged from systematic studies over the last two decades; in this way, we hope to advance the field of peptide and protein engineering based on the judicious introduction of fluorinated building blocks.
中文翻译:

解读氟代码—在蛋白质环境中戴许多帽子氟
解密氟代码不仅是我们描述该帐户的重点,而且是研究氟掺入对我们小组及其他人员使用的肽和蛋白质性质的影响的系统方法。氟的引入已显示出赋予肽和蛋白质有利的但很少可预测的性质,但是直到大约二十年前,肽和蛋白质的氟修饰的结果很大程度上尚待商chance。在将氟元素的独特性质从医学和农业化学扩展到肽和蛋白质工程的动机的驱使下,我们建立了广泛的研究计划,使能够系统地研究将氟引入此类生物聚合物中的作用。 。将氟引入氨基酸为修饰氨基酸侧链中的氟取代基的数量和位置提供了多种选择。此外,必须强调的是,将C–F键掺入生物聚合物的后果可归因于两个截然不同但相关的现象:(i)氟取代基可与其环境直接发生分子间相互作用,和/或(ii )分子中存在的其他官能团会受到该元素(分子内)的吸电子性质的影响,进而与其周围环境(分子间)发生不同的相互作用。根据我们的研究,我们已经表明,数量和/或位置的变化与单个氟取代基一样微妙,具有显着改变氨基酸的关键特性(例如疏水性,极性和二级结构倾向)的能力。这些特性是肽和蛋白质工程中的关键因素,因此,只要我们了解并能够预测蛋白质的折叠结果,氟化氨基酸就可以用于微调特性,例如蛋白质折叠,蛋白水解稳定性和蛋白质-蛋白质相互作用。在这种情况下,氟取代。通过这个帐户,我们尝试分析从最近的项目中获得的信息,这些信息涉及氟原子和C–F键的性质如何影响肽和蛋白质的四个关键特性:肽折叠,蛋白质-蛋白质相互作用,核糖体翻译,和蛋白酶的稳定性。这些结果令人印象深刻地说明了为什么引入氟会产生一类新的具有各种功能的氨基酸,这种功能是蛋白质界所独有的,并且在某些情况下与一组规范的和天然的氨基酸正交。我们的结论性声明旨在提供在过去的二十年中从系统研究中得出的一些保守的设计原则。通过这种方式,我们希望在明智地引入氟化结构单元的基础上,推动肽和蛋白质工程领域的发展。我们的结论性声明旨在提供在过去的二十年中从系统研究中得出的一些保守的设计原则。通过这种方式,我们希望在明智地引入氟化结构单元的基础上,推动肽和蛋白质工程领域的发展。我们的结论性声明旨在提供在过去的二十年中从系统研究中得出的一些保守的设计原则。通过这种方式,我们希望在明智地引入氟化结构单元的基础上,推动肽和蛋白质工程领域的发展。
更新日期:2017-08-12
中文翻译:

解读氟代码—在蛋白质环境中戴许多帽子氟
解密氟代码不仅是我们描述该帐户的重点,而且是研究氟掺入对我们小组及其他人员使用的肽和蛋白质性质的影响的系统方法。氟的引入已显示出赋予肽和蛋白质有利的但很少可预测的性质,但是直到大约二十年前,肽和蛋白质的氟修饰的结果很大程度上尚待商chance。在将氟元素的独特性质从医学和农业化学扩展到肽和蛋白质工程的动机的驱使下,我们建立了广泛的研究计划,使能够系统地研究将氟引入此类生物聚合物中的作用。 。将氟引入氨基酸为修饰氨基酸侧链中的氟取代基的数量和位置提供了多种选择。此外,必须强调的是,将C–F键掺入生物聚合物的后果可归因于两个截然不同但相关的现象:(i)氟取代基可与其环境直接发生分子间相互作用,和/或(ii )分子中存在的其他官能团会受到该元素(分子内)的吸电子性质的影响,进而与其周围环境(分子间)发生不同的相互作用。根据我们的研究,我们已经表明,数量和/或位置的变化与单个氟取代基一样微妙,具有显着改变氨基酸的关键特性(例如疏水性,极性和二级结构倾向)的能力。这些特性是肽和蛋白质工程中的关键因素,因此,只要我们了解并能够预测蛋白质的折叠结果,氟化氨基酸就可以用于微调特性,例如蛋白质折叠,蛋白水解稳定性和蛋白质-蛋白质相互作用。在这种情况下,氟取代。通过这个帐户,我们尝试分析从最近的项目中获得的信息,这些信息涉及氟原子和C–F键的性质如何影响肽和蛋白质的四个关键特性:肽折叠,蛋白质-蛋白质相互作用,核糖体翻译,和蛋白酶的稳定性。这些结果令人印象深刻地说明了为什么引入氟会产生一类新的具有各种功能的氨基酸,这种功能是蛋白质界所独有的,并且在某些情况下与一组规范的和天然的氨基酸正交。我们的结论性声明旨在提供在过去的二十年中从系统研究中得出的一些保守的设计原则。通过这种方式,我们希望在明智地引入氟化结构单元的基础上,推动肽和蛋白质工程领域的发展。我们的结论性声明旨在提供在过去的二十年中从系统研究中得出的一些保守的设计原则。通过这种方式,我们希望在明智地引入氟化结构单元的基础上,推动肽和蛋白质工程领域的发展。我们的结论性声明旨在提供在过去的二十年中从系统研究中得出的一些保守的设计原则。通过这种方式,我们希望在明智地引入氟化结构单元的基础上,推动肽和蛋白质工程领域的发展。


























 京公网安备 11010802027423号
京公网安备 11010802027423号