Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2017-08-12 , DOI: 10.1016/j.apcata.2017.08.013 Jingbo Jia , Wenjuan Yang , Pengyi Zhang , Junying Zhang
|
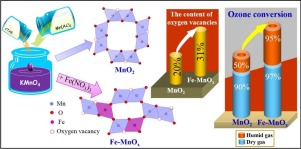
Oxygen vacancy engineering is an efficient strategy to improve the catalytic performance of nanomaterials. In this work, a highly active Fe-modified manganese oxide (Fe-MnOx) was synthesized and used for airborne ozone decomposition. The addition of Fe3+ during MnO2 synthesis led to higher specific surface area, greatly increased content of oxygen vacancies, evidenced by XPS, H2-TPR analysis and lower oxygen vacancy formation energy (decreased by ∼1.2 eV) based on the density functional theory calculations. The ozone conversion over Fe-MnOx kept 97% after 24 h reaction, while it over MnO2 slowed down to 85% under dry condition. Remarkably, under humid condition (RH = 60%), the ozone conversion over Fe-MnOx kept 73% after 6 h reaction, while ozone conversion over pure MnO2 decreased to 50% within 1 h under the conditions of 100 ppm inlet ozone concentration and weight space velocity of 660 L g−1 h−1. The intermediate peroxide species (O22−) formed on the surface oxygen vacancies of Fe-MnOx and MnO2 during ozone decomposition reaction were observed using in situ Raman spectroscopy. The concentration and depletion rate of O22− on the surface of Fe-MnOx was higher than that on MnO2, illustrating that O22− acted as the key species to boost the catalytic process. The content and dispersity of oxygen vacancies were identified to be mainly responsible for the performance difference. This provides a promising idea for designing novel nanomaterial catalyst for gaseous ozone decomposition.
中文翻译:

容易合成具有高氧空缺含量的铁修饰的锰氧化物,可有效清除空气中的臭氧
氧空位工程是提高纳米材料催化性能的有效策略。在这项工作中,合成了高活性的铁改性的锰氧化物(Fe-MnO x),并用于空气中的臭氧分解。通过XPS,H 2 -TPR分析证明,在MnO 2合成过程中添加Fe 3+会导致更高的比表面积,氧空位含量大大增加以及基于密度的更低的氧空位形成能(降低约1.2 eV)。功能理论计算。反应24小时后,Fe-MnO x上的臭氧转化率保持97%,而MnO 2上的臭氧转化率保持在干燥条件下减慢至85%。值得注意的是,在潮湿条件下(RH = 60%),反应6小时后,Fe-MnO x上的臭氧转化率保持73%,而纯MnO 2上的臭氧转化率在100 ppm进口臭氧的条件下在1小时内降至50%。浓度和660 L g -1 h -1的重空间速度。中间过氧化物物质(O 2 2-),形成在铁基的MnO的表面氧空位X和MnO 2臭氧分解反应期间使用,观察到原位拉曼光谱。Fe-MnO x表面上O 2 2-的浓度和耗竭率高于MnO 2,说明O 2 2-是促进催化过程的关键物质。确定氧空位的含量和分散性是造成性能差异的主要原因。这为设计用于气态臭氧分解的新型纳米材料催化剂提供了一个有希望的想法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号