当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preparation of the HIV Attachment Inhibitor BMS-663068. Part 3. Mechanistic Studies Enable a Scale-Independent Friedel–Crafts Acylation
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00115 Gregory L. Beutner 1 , Jacob Albrecht 1 , Junying Fan 1 , Dayne Fanfair 1 , Michael J. Lawler 1 , Michael Bultman 1 , Ke Chen 1 , Sabrina Ivy 1 , Richard L. Schild 1 , Jonathan C. Tripp 1 , Saravanababu Murugesan 1 , Konstantinos Dambalas 1 , Douglas D. McLeod 1 , Jason T. Sweeney 1 , Martin D. Eastgate 1 , David A. Conlon 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00115 Gregory L. Beutner 1 , Jacob Albrecht 1 , Junying Fan 1 , Dayne Fanfair 1 , Michael J. Lawler 1 , Michael Bultman 1 , Ke Chen 1 , Sabrina Ivy 1 , Richard L. Schild 1 , Jonathan C. Tripp 1 , Saravanababu Murugesan 1 , Konstantinos Dambalas 1 , Douglas D. McLeod 1 , Jason T. Sweeney 1 , Martin D. Eastgate 1 , David A. Conlon 1
Affiliation
|
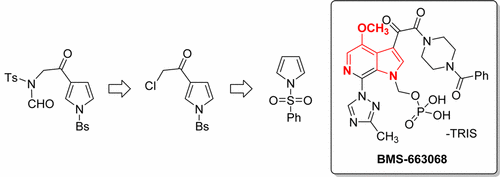
During the development of a Friedel–Crafts acylation for the preparation of a key pyrrole intermediate in the synthesis of the HIV attachment inhibitor, BMS-663068-03, a significant scale dependence was found. A precipitous drop in yield was observed for the acylation of a protected pyrrole with chloroacetyl chloride upon scale-up. Spectroscopic studies to mitigate this scale dependence led to the identification of the complex effect of dissolved hydrogen chloride (HCl) as well as the poor reactivity of the acylating agent, chloroacetyl chloride. At this point, the counterintuitive choice to switch to a longer, but scale-independent, three-step route was made. By changing the acylating agent to acetyl chloride, a more robust process was obtained. Rapid development of a high yielding α-chlorination then provided the common α-chloroketone intermediate required to generate the desired α-amide ketopyrrole. The improved yield and scalability of this three-step process supported the addition of one linear step to the route, and it was demonstrated successfully at scale.
中文翻译:

HIV附着抑制剂BMS-663068的制备。第3部分。机理研究实现了与标度无关的Friedel-Crafts酰化作用
在开发用于合成HIV附着抑制剂BMS-663068-03的关键吡咯中间体的Friedel-Crafts酰化技术的开发过程中,发现了显着的规模依赖性。观察到规模放大后,用氯乙酰氯酰化保护的吡咯的收率急剧下降。为减轻这种规模依赖性而进行的光谱研究导致对溶解的氯化氢(HCl)的复杂作用以及酰化剂氯乙酰氯的不良反应性的鉴定。在这一点上,做出了违反直觉的选择,即切换到更长但与规模无关的三步走路线。通过将酰化剂改变为乙酰氯,获得了更稳定的方法。然后迅速发展出高产的α-氯化物,提供了产生所需α-酰胺酮吡咯所需的普通α-氯酮中间体。此三步过程的改进的产量和可扩展性支持在路线上增加一个线性步,并且已成功地进行了规模论证。
更新日期:2017-08-09
中文翻译:

HIV附着抑制剂BMS-663068的制备。第3部分。机理研究实现了与标度无关的Friedel-Crafts酰化作用
在开发用于合成HIV附着抑制剂BMS-663068-03的关键吡咯中间体的Friedel-Crafts酰化技术的开发过程中,发现了显着的规模依赖性。观察到规模放大后,用氯乙酰氯酰化保护的吡咯的收率急剧下降。为减轻这种规模依赖性而进行的光谱研究导致对溶解的氯化氢(HCl)的复杂作用以及酰化剂氯乙酰氯的不良反应性的鉴定。在这一点上,做出了违反直觉的选择,即切换到更长但与规模无关的三步走路线。通过将酰化剂改变为乙酰氯,获得了更稳定的方法。然后迅速发展出高产的α-氯化物,提供了产生所需α-酰胺酮吡咯所需的普通α-氯酮中间体。此三步过程的改进的产量和可扩展性支持在路线上增加一个线性步,并且已成功地进行了规模论证。

























 京公网安备 11010802027423号
京公网安备 11010802027423号