当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preparation of the HIV Attachment Inhibitor BMS-663068. Part 8. Installation of the Phosphonoxymethyl Prodrug Moiety
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00135 Richard J. Fox 1 , Benjamin Cohen 1 , Thomas E. La Cruz 1 , James H. Simpson 1 , Adam Freitag 1 , Eric Saurer 1 , Jonathan C. Tripp 1 , Chien-Kuang Chen 1 , Gregory L. Beutner 1 , Victor W. Rosso 1 , Elizabeth Borgeson 1 , Andrew W. Glace 1 , Martin D. Eastgate 1 , Richard L. Schild 1 , Jason T. Sweeney 1 , David A. Conlon 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00135 Richard J. Fox 1 , Benjamin Cohen 1 , Thomas E. La Cruz 1 , James H. Simpson 1 , Adam Freitag 1 , Eric Saurer 1 , Jonathan C. Tripp 1 , Chien-Kuang Chen 1 , Gregory L. Beutner 1 , Victor W. Rosso 1 , Elizabeth Borgeson 1 , Andrew W. Glace 1 , Martin D. Eastgate 1 , Richard L. Schild 1 , Jason T. Sweeney 1 , David A. Conlon 1
Affiliation
|
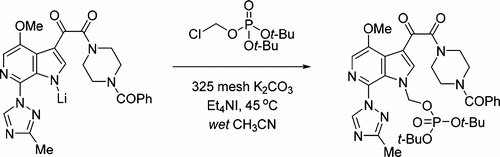
The reaction optimization of an alkylation to enable the production of the penultimate intermediate of an HIV-attachment inhibitor candidate is described. To address the challenges associated with the reactivity and stability of di-tert-butyl (chloromethyl) phosphate (2), and the poor solubility and reactivity of the starting BMS-626529-Li salt (1), strategic selection of Et4NI and 325 mesh K2CO3 as additives, and wet CH3CN as solvent were required. An aqueous workup protocol was also developed to selectively remove an undesired N-6 alkylation isomer. The final processing conditions resulted in the isolation of the penultimate compound 3 in 70% yield with high purity.
中文翻译:

HIV附着抑制剂BMS-663068的制备。第8部分。膦氧甲基前药部分的安装
描述了烷基化的反应优化,以使得能够产生HIV-附着抑制剂候选物的倒数第二个中间体。为了解决与反应性和二-稳定性相关的挑战叔丁基(氯甲基)酯(2),以及差的溶解性和反应性起始BMS-626529-Li盐(1),等的战略选择4 NI和需要325目K 2 CO 3作为添加剂,并且需要湿CH 3 CN作为溶剂。还开发了水性处理方案以选择性除去不希望的N-6烷基化异构体。最终加工条件导致分离出倒数第二个化合物3 收率高达70%,纯度高。
更新日期:2017-08-09
中文翻译:

HIV附着抑制剂BMS-663068的制备。第8部分。膦氧甲基前药部分的安装
描述了烷基化的反应优化,以使得能够产生HIV-附着抑制剂候选物的倒数第二个中间体。为了解决与反应性和二-稳定性相关的挑战叔丁基(氯甲基)酯(2),以及差的溶解性和反应性起始BMS-626529-Li盐(1),等的战略选择4 NI和需要325目K 2 CO 3作为添加剂,并且需要湿CH 3 CN作为溶剂。还开发了水性处理方案以选择性除去不希望的N-6烷基化异构体。最终加工条件导致分离出倒数第二个化合物3 收率高达70%,纯度高。


























 京公网安备 11010802027423号
京公网安备 11010802027423号