当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preparation of the HIV Attachment Inhibitor BMS-663068. Part 7. Development of a Regioselective Ullmann–Goldberg–Buchwald Reaction
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00191 William P. Gallagher 1 , Maxime Soumeillant 1 , Ke Chen 1 , Richard J. Fox 1 , Yi Hsiao 1 , Brendan Mack 1 , Vidya Iyer 1 , Junying Fan 1 , Jason Zhu 1 , Gregory Beutner 1 , Steven M. Silverman 1 , Dayne D. Fanfair 1 , Andrew W. Glace 1 , Adam Freitag 1 , Jason Sweeney 1 , Yining Ji 2 , Donna G. Blackmond 2 , Martin D. Eastgate 1 , David A. Conlon 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-08-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00191 William P. Gallagher 1 , Maxime Soumeillant 1 , Ke Chen 1 , Richard J. Fox 1 , Yi Hsiao 1 , Brendan Mack 1 , Vidya Iyer 1 , Junying Fan 1 , Jason Zhu 1 , Gregory Beutner 1 , Steven M. Silverman 1 , Dayne D. Fanfair 1 , Andrew W. Glace 1 , Adam Freitag 1 , Jason Sweeney 1 , Yining Ji 2 , Donna G. Blackmond 2 , Martin D. Eastgate 1 , David A. Conlon 1
Affiliation
|
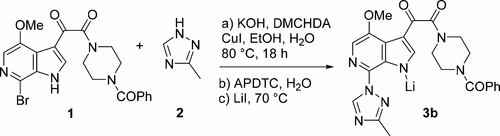
The discovery, development, and optimization of an Ullmann–Goldberg–Buchwald coupling reaction is described. This complex process represents a key transformation in the development of a commercially viable synthesis of the HIV attachment inhibitor prodrug BMS-663068. In this reaction, high regioselectivities were obtained for the coupling of a 1,2,4-triazole and a 7-bromoazaindole, preparing BMS-626529, the antepenultimate in good yield and quality. Key challenges associated with developing commercially viable conditions for this copper-mediated coupling include achieving the desired level of regiochemical control, identifying robust isolation conditions and controlling residual copper levels in the isolated product.
中文翻译:

HIV附着抑制剂BMS-663068的制备。第7部分。区域选择性乌尔曼-戈德堡-布赫瓦尔德反应的发展
描述了Ullmann-Goldberg-Buchwald偶联反应的发现,开发和优化。这个复杂的过程代表了HIV附着抑制剂前药BMS-663068在商业上可行的合成方法开发中的关键转变。在该反应中,对于1,2,4-三唑和7-溴氮杂吲哚的偶联,获得了高区域选择性,从而制备了BMS-626529,其收率和质量都很高。为此铜介导的偶联物开发商业上可行的条件所面临的主要挑战包括实现所需的区域化学控制水平,确定可靠的分离条件并控制分离产物中的残留铜水平。
更新日期:2017-08-09
中文翻译:

HIV附着抑制剂BMS-663068的制备。第7部分。区域选择性乌尔曼-戈德堡-布赫瓦尔德反应的发展
描述了Ullmann-Goldberg-Buchwald偶联反应的发现,开发和优化。这个复杂的过程代表了HIV附着抑制剂前药BMS-663068在商业上可行的合成方法开发中的关键转变。在该反应中,对于1,2,4-三唑和7-溴氮杂吲哚的偶联,获得了高区域选择性,从而制备了BMS-626529,其收率和质量都很高。为此铜介导的偶联物开发商业上可行的条件所面临的主要挑战包括实现所需的区域化学控制水平,确定可靠的分离条件并控制分离产物中的残留铜水平。


























 京公网安备 11010802027423号
京公网安备 11010802027423号