当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metal–organic gels of silver salts with an α,β-unsaturated ketone: the influence of anions and solvents on gelation
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2017-06-26 00:00:00 , DOI: 10.1039/c7qi00328e Debarati Das 1, 2, 3, 4 , Kumar Biradha 1, 2, 3, 4
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2017-06-26 00:00:00 , DOI: 10.1039/c7qi00328e Debarati Das 1, 2, 3, 4 , Kumar Biradha 1, 2, 3, 4
Affiliation
|
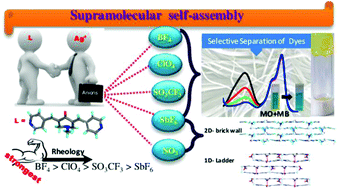
A bis-pyridyl substituted α,β-unsaturated ketone, namely 1-methyl-3,5-bis-pyridin-4-ylmethylene-piperidin-4-one, L, was shown to form metal organic gels (MOGs) with silver salts. The presence of anions such as tetrafluoroborate, perchlorate, triflate and hexafluoroantimonate resulted in the formation of MOGs in various solvents. The MOGs exhibit well-developed nanofibrillar networks composed of intertwined fibers and are characterized by field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), Fourier transform infrared (FT-IR) spectroscopy, rheology and powder X-ray diffraction (PXRD) techniques. The gelation was studied in as many as twenty organic solvents. Among all the solvents used, benzonitrile was found to promote the gelation of L with as many as four silver salts, namely BF4 (MOG-1), ClO4 (MOG-2), CF3SO3 (MOG-3) and SbF6 (MOG-4). All these xerogels were found to be polycrystalline in nature as confirmed by PXRD. FESEM images of the xerogel of MOG-2 revealed that it exhibits spherical morphology consisting of fibrils. Rheological studies indicate that MOG-1 is the strongest gel among all four and the rigidity order follows BF4 > ClO4 > CF3SO3 > SbF6. Furthermore, the xerogels were found to exhibit selective dye adsorption and separation. The information on interactions and structures involved in the gelation of solvents was obtained by the single crystal structure determination of Ag(I) complexes of L with NO3 and SbF6 anions.
中文翻译:

具有α,β-不饱和酮的银盐的金属有机凝胶:阴离子和溶剂对凝胶化的影响
已显示双吡啶基取代的α,β-不饱和酮(即1-甲基-3,5-双吡啶-4-基亚甲基-哌啶-4-酮L)与银盐形成金属有机凝胶(MOG) 。阴离子(如四氟硼酸根,高氯酸根,三氟甲磺酸根和六氟锑酸根)的存在导致在各种溶剂中形成MOG。MOG展现出由缠结纤维组成的发达的纳米原纤维网络,并具有场发射扫描电子显微镜(FESEM),透射电子显微镜(TEM),傅立叶变换红外(FT-IR)光谱,流变学和粉末X射线衍射( PXRD)技术。在多达二十种有机溶剂中研究了凝胶化。在所有使用的溶剂中,发现苄腈可促进L的胶凝含有多达四种银盐,分别是BF 4(MOG- 1),ClO 4(MOG- 2),CF 3 SO 3(MOG- 3)和SbF 6(MOG- 4)。如PXRD所证实的,发现所有这些干凝胶本质上都是多晶的。MOG- 2的干凝胶的FESEM图像显示,它显示出由原纤维组成的球形形态。流变学研究表明,MOG- 1是所有四种凝胶中最强的凝胶,其刚性顺序依次为BF 4 > ClO 4 > CF 3 SO 3 > SbF 6。此外,发现干凝胶表现出选择性的染料吸附和分离。通过单晶结构测定L与NO 3和SbF 6阴离子形成的Ag(I)配合物,可以获得与溶剂凝胶化有关的相互作用和结构的信息。
更新日期:2017-08-08
中文翻译:

具有α,β-不饱和酮的银盐的金属有机凝胶:阴离子和溶剂对凝胶化的影响
已显示双吡啶基取代的α,β-不饱和酮(即1-甲基-3,5-双吡啶-4-基亚甲基-哌啶-4-酮L)与银盐形成金属有机凝胶(MOG) 。阴离子(如四氟硼酸根,高氯酸根,三氟甲磺酸根和六氟锑酸根)的存在导致在各种溶剂中形成MOG。MOG展现出由缠结纤维组成的发达的纳米原纤维网络,并具有场发射扫描电子显微镜(FESEM),透射电子显微镜(TEM),傅立叶变换红外(FT-IR)光谱,流变学和粉末X射线衍射( PXRD)技术。在多达二十种有机溶剂中研究了凝胶化。在所有使用的溶剂中,发现苄腈可促进L的胶凝含有多达四种银盐,分别是BF 4(MOG- 1),ClO 4(MOG- 2),CF 3 SO 3(MOG- 3)和SbF 6(MOG- 4)。如PXRD所证实的,发现所有这些干凝胶本质上都是多晶的。MOG- 2的干凝胶的FESEM图像显示,它显示出由原纤维组成的球形形态。流变学研究表明,MOG- 1是所有四种凝胶中最强的凝胶,其刚性顺序依次为BF 4 > ClO 4 > CF 3 SO 3 > SbF 6。此外,发现干凝胶表现出选择性的染料吸附和分离。通过单晶结构测定L与NO 3和SbF 6阴离子形成的Ag(I)配合物,可以获得与溶剂凝胶化有关的相互作用和结构的信息。



























 京公网安备 11010802027423号
京公网安备 11010802027423号