当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereochemistry of a Second Riolozane and Other Diterpenoids from Jatropha dioica
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-08-03 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00193 Elda M. Melchor-Martínez 1, 2 , David A. Silva-Mares 1 , Ernesto Torres-López 1 , Noemí Waksman-Minsky 1 , Guido F. Pauli 3 , Shao-Nong Chen 3 , Matthias Niemitz 4 , Mariano Sánchez-Castellanos 2 , Alfredo Toscano 2 , Gabriel Cuevas 2 , Verónica M. Rivas-Galindo 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-08-03 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00193 Elda M. Melchor-Martínez 1, 2 , David A. Silva-Mares 1 , Ernesto Torres-López 1 , Noemí Waksman-Minsky 1 , Guido F. Pauli 3 , Shao-Nong Chen 3 , Matthias Niemitz 4 , Mariano Sánchez-Castellanos 2 , Alfredo Toscano 2 , Gabriel Cuevas 2 , Verónica M. Rivas-Galindo 1
Affiliation
|
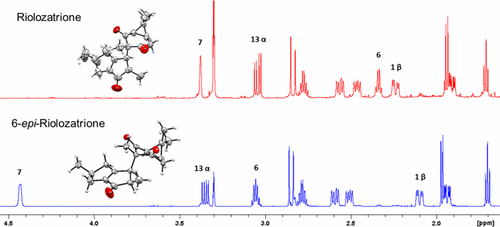
The dichloromethane extract of the roots of Jatropha dioica afforded riolozatrione (1) and a C-6 epimer of riolozatrione, 6-epi-riolozatrione (2), as a new structure and only the second reported riolozane diterpenoid. The two known diterpenoids jatrophatrione (3) and citlalitrione (4) were also isolated and characterized. Both epimers 1 and 2 are genuine plant constituents, with 2 likely being the biosynthesis precursor of 1 due to the tendency for the quantitative transformation of 2 into 1 under base catalysis. The structural characterization and distinction of the stereoisomers utilized 1H iterative full-spin analysis, yielding complete J-correlation maps that were represented as quantum interaction and linkage tables. The absolute configuration of compounds 1–4 was established by means of vibrational circular dichroism and via X-ray diffraction analysis for 1, 2, and 4. Additionally, the cytotoxic and antiherpetic in vitro activities of the isolates were evaluated.
中文翻译:

麻风树麻疯树中第二个氟氮烷和其他二萜类化合物的立体化学
麻风树根的二氯甲烷提取物提供了新的结构,并提供了新的结构,并提供了新的结构,并提供了新的结构,其中包括了riolozatrione(1)和C-6异构体,riolozatrione(6- epi- riolozatrione)(2)。还分离并表征了两种已知的二萜类化合物麻风三酮(3)和瓜三酮(4)。差向异构体二者1和2是真正的植物成分,用2可能是生物合成的前体1由于对的定量转化的倾向2到1在碱催化下。立体异构体的结构表征和区别使用1 H迭代全自旋分析,产生完整的J相关图,这些图表示为量子相互作用和键合表。化合物的绝对构型1 - 4通过振动圆二色的装置和经由用于X射线衍射分析,建立1,2,和4。另外,评估了分离物的细胞毒性和抗疱疹性体外活性。
更新日期:2017-08-03
中文翻译:

麻风树麻疯树中第二个氟氮烷和其他二萜类化合物的立体化学
麻风树根的二氯甲烷提取物提供了新的结构,并提供了新的结构,并提供了新的结构,并提供了新的结构,其中包括了riolozatrione(1)和C-6异构体,riolozatrione(6- epi- riolozatrione)(2)。还分离并表征了两种已知的二萜类化合物麻风三酮(3)和瓜三酮(4)。差向异构体二者1和2是真正的植物成分,用2可能是生物合成的前体1由于对的定量转化的倾向2到1在碱催化下。立体异构体的结构表征和区别使用1 H迭代全自旋分析,产生完整的J相关图,这些图表示为量子相互作用和键合表。化合物的绝对构型1 - 4通过振动圆二色的装置和经由用于X射线衍射分析,建立1,2,和4。另外,评估了分离物的细胞毒性和抗疱疹性体外活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号