PLOS Medicine ( IF 15.8 ) Pub Date : 2017-07-17 , DOI: 10.1371/journal.pmed.1002352 Claudia P Cabrera 1 , Joanna Manson 2 , Joanna M Shepherd 2 , Hew D Torrance 3 , David Watson 1 , M Paula Longhi 4 , Mimoza Hoti 5 , Minal B Patel 1 , Michael O'Dwyer 3 , Sussan Nourshargh 6 , Daniel J Pennington 7 , Michael R Barnes 1 , Karim Brohi 2
|
Background
Severe trauma induces a widespread response of the immune system. This “genomic storm” can lead to poor outcomes, including Multiple Organ Dysfunction Syndrome (MODS). MODS carries a high mortality and morbidity rate and adversely affects long-term health outcomes. Contemporary management of MODS is entirely supportive, and no specific therapeutics have been shown to be effective in reducing incidence or severity. The pathogenesis of MODS remains unclear, and several models are proposed, such as excessive inflammation, a second-hit insult, or an imbalance between pro- and anti-inflammatory pathways. We postulated that the hyperacute window after trauma may hold the key to understanding how the genomic storm is initiated and may lead to a new understanding of the pathogenesis of MODS.
Methods and findings
We performed whole blood transcriptome and flow cytometry analyses on a total of 70 critically injured patients (Injury Severity Score [ISS] ≥ 25) at The Royal London Hospital in the hyperacute time period within 2 hours of injury. We compared transcriptome findings in 36 critically injured patients with those of 6 patients with minor injuries (ISS ≤ 4). We then performed flow cytometry analyses in 34 critically injured patients and compared findings with those of 9 healthy volunteers. Immediately after injury, only 1,239 gene transcripts (4%) were differentially expressed in critically injured patients. By 24 hours after injury, 6,294 transcripts (21%) were differentially expressed compared to the hyperacute window. Only 202 (16%) genes differentially expressed in the hyperacute window were still expressed in the same direction at 24 hours postinjury. Pathway analysis showed principally up-regulation of pattern recognition and innate inflammatory pathways, with down-regulation of adaptive responses. Immune deconvolution, flow cytometry, and modular analysis suggested a central role for neutrophils and Natural Killer (NK) cells, with underexpression of T- and B cell responses.
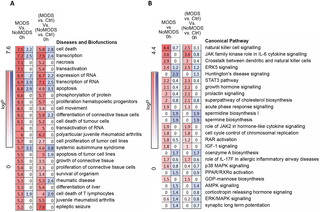
In the transcriptome cohort, 20 critically injured patients later developed MODS. Compared with the 16 patients who did not develop MODS (NoMODS), maximal differential expression was seen within the hyperacute window. In MODS versus NoMODS, 363 genes were differentially expressed on admission, compared to only 33 at 24 hours postinjury. MODS transcripts differentially expressed in the hyperacute window showed enrichment among diseases and biological functions associated with cell survival and organismal death rather than inflammatory pathways. There was differential up-regulation of NK cell signalling pathways and markers in patients who would later develop MODS, with down-regulation of neutrophil deconvolution markers. This study is limited by its sample size, precluding more detailed analyses of drivers of the hyperacute response and different MODS phenotypes, and requires validation in other critically injured cohorts.
Conclusions
In this study, we showed how the hyperacute postinjury time window contained a focused, specific signature of the response to critical injury that led to widespread genomic activation. A transcriptomic signature for later development of MODS was present in this hyperacute window; it showed a strong signal for cell death and survival pathways and implicated NK cells and neutrophil populations in this differential response.
中文翻译:

创伤超急性期炎症的特征和即将发生的多器官功能障碍:一项前瞻性队列研究
背景
严重的创伤会引起免疫系统的广泛反应。这种“基因组风暴”可能导致不良结果,包括多器官功能障碍综合征 (MODS)。MODS 具有很高的死亡率和发病率,并对长期健康结果产生不利影响。MODS 的现代管理是完全支持性的,并且没有特定的治疗方法被证明可有效降低发病率或严重程度。MODS 的发病机制仍不清楚,并且提出了几种模型,例如过度炎症、二次损伤或促炎和抗炎途径之间的不平衡。我们假设创伤后的超急性窗口可能是了解基因组风暴如何启动的关键,并可能导致对 MODS 发病机制的新认识。
方法和发现
我们在受伤后 2 小时内的超急性期对伦敦皇家医院的 70 名重症患者(损伤严重程度评分 [ISS] ≥ 25)进行了全血转录组和流式细胞术分析。我们比较了 36 名重伤患者和 6 名轻伤患者(ISS ≤ 4)的转录组结果。然后,我们对 34 名严重受伤的患者进行了流式细胞术分析,并将结果与 9 名健康志愿者的结果进行了比较。受伤后,只有 1,239 个基因转录本 (4%) 在重伤患者中差异表达。到损伤后 24 小时,与超急性窗口相比,有 6,294 个转录本 (21%) 有差异表达。只有 202 (16%) 个在超急性窗口中差异表达的基因在损伤后 24 小时仍以相同方向表达。通路分析主要显示模式识别和先天炎症通路上调,适应性反应下调。免疫反卷积、流式细胞术和模块化分析表明中性粒细胞和自然杀伤 (NK) 细胞具有核心作用,但 T 细胞和 B 细胞反应的表达不足。
在转录组队列中,20 名重伤患者后来发展为 MODS。与未发生 MODS (NoMODS) 的 16 名患者相比,在超急性窗口内观察到最大差异表达。在 MODS 与 NoMODS 中,363 个基因在入院时有差异表达,而受伤后 24 小时只有 33 个。在超急性窗口中差异表达的 MODS 转录本显示出与细胞存活和有机体死亡相关的疾病和生物学功能的富集,而不是炎症通路。在后来发展为 MODS 的患者中,NK 细胞信号通路和标志物存在差异上调,而中性粒细胞去卷积标志物则下调。本研究受限于样本量,
结论
在这项研究中,我们展示了超急性损伤后时间窗口如何包含对导致广泛基因组激活的严重损伤的反应的集中、特异性特征。在这个超急性窗口中出现了后来发展为 MODS 的转录组学特征。它显示了细胞死亡和存活途径的强烈信号,并暗示 NK 细胞和中性粒细胞群体参与了这种差异反应。

























 京公网安备 11010802027423号
京公网安备 11010802027423号