当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrophilic Triterpenoid Enones: A Comparative Thiol-Trapping and Bioactivity Study
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-07-28 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00271 Danilo Del Prete 1 , Orazio Taglialatela-Scafati 2 , Alberto Minassi 1 , Carmina Sirignano 2 , Cristina Cruz 3 , Maria L Bellido 3 , Eduardo Muñoz 4 , Giovanni Appendino 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-07-28 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00271 Danilo Del Prete 1 , Orazio Taglialatela-Scafati 2 , Alberto Minassi 1 , Carmina Sirignano 2 , Cristina Cruz 3 , Maria L Bellido 3 , Eduardo Muñoz 4 , Giovanni Appendino 1
Affiliation
|
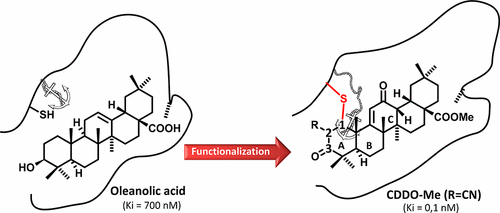
Bardoxolone methyl (1) is the quintessential member of triterpenoid cyanoacrylates, an emerging class of bioactive compounds capable of transient covalent binding to thiols. The mechanistic basis for this unusual “pulsed reactivity” profile and the mode of its biological translation are unknown. To provide clues on these issues, a series of Δ1-dehydrooleanolates bearing an electron-withdrawing group at C-2 (7a–m) were prepared from oleanolic acid (3a) and comparatively investigated in terms of reactivity with thiols and bioactivity against a series of electrophile-sensitive transcription factors (Nrf2, NF-κB, STAT3). The emerging picture suggests that the triterpenoid scaffold sharply decreases the reactivity of the enone system by steric encumbrance and that only strongly electrophilic and sterically undemanding substituents such as a cyanide or a carboxylate group can re-establish Michael reactivity, albeit in a transient way for the cyanide group. In general, a substantial dissection between the thiol-trapping ability and the modulation of biological end-points sensitive to thiol alkylation was observed, highlighting the role of shape complementarity for the activity of triterpenoid thia-Michael acceptors.
中文翻译:

亲电子三萜烯酮:硫醇诱捕和生物活性的比较研究。
Bardoxolone甲基(1)是三萜氰基丙烯酸酯的典型成员,这是一类新兴的能够与硫醇瞬时共价结合的生物活性化合物。这种异常的“脉冲反应性”特征的机理基础及其生物学翻译的模式尚不清楚。为了提供对这些问题的线索,一系列的Δ 1 -dehydrooleanolates轴承在C-2的吸电子基团(7a中-米)从齐墩果酸制备(图3a),并在与硫醇的反应性和对一系列亲电敏感的转录因子(Nrf2,NF-κB,STAT3)的生物活性方面进行了比较研究。新兴的图片表明,三萜骨架通过空间阻碍显着降低了烯酮体系的反应性,并且只有强亲电和空间上不需要的取代基(如氰化物或羧酸酯基团)才能重新建立迈克尔反应性,尽管这是一种短暂的方式。氰化物基团。通常,观察到在硫醇捕获能力和对硫醇烷基化敏感的生物端点的调节之间的实质分离,突出了形状互补性对三萜类噻吩-迈克尔受体活性的作用。
更新日期:2017-07-28
中文翻译:

亲电子三萜烯酮:硫醇诱捕和生物活性的比较研究。
Bardoxolone甲基(1)是三萜氰基丙烯酸酯的典型成员,这是一类新兴的能够与硫醇瞬时共价结合的生物活性化合物。这种异常的“脉冲反应性”特征的机理基础及其生物学翻译的模式尚不清楚。为了提供对这些问题的线索,一系列的Δ 1 -dehydrooleanolates轴承在C-2的吸电子基团(7a中-米)从齐墩果酸制备(图3a),并在与硫醇的反应性和对一系列亲电敏感的转录因子(Nrf2,NF-κB,STAT3)的生物活性方面进行了比较研究。新兴的图片表明,三萜骨架通过空间阻碍显着降低了烯酮体系的反应性,并且只有强亲电和空间上不需要的取代基(如氰化物或羧酸酯基团)才能重新建立迈克尔反应性,尽管这是一种短暂的方式。氰化物基团。通常,观察到在硫醇捕获能力和对硫醇烷基化敏感的生物端点的调节之间的实质分离,突出了形状互补性对三萜类噻吩-迈克尔受体活性的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号