当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solvent-Selective Reactions of Alkyl Iodide with Sodium Azide for Radical Generation and Azide Substitution and Their Application to One-Pot Synthesis of Chain-End-Functionalized Polymers
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-07-25 , DOI: 10.1021/jacs.7b05879 Chen-Gang Wang 1 , Atsushi Goto 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-07-25 , DOI: 10.1021/jacs.7b05879 Chen-Gang Wang 1 , Atsushi Goto 1
Affiliation
|
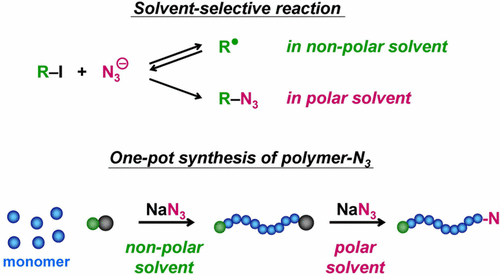
Herein, a new reaction of an alkyl iodide (R-I) with an azide anion (N3-) to reversibly generate the corresponding alkyl radical (R•) is reported. Via this new reaction, N3- was used as an efficient catalyst in living radical polymerization, yielding a well-defined polymer-iodide. A particularly interesting finding was the solvent selectivity of this reaction; namely, R-I and N3- generated R• in nonpolar solvents, while the substitution product R-N3 was generated in polar solvents. Exploiting this unique solvent selectivity, a one-pot synthesis of polymer-N3 was attained. N3- was first used as a catalyst for living radical polymerization in a nonpolar solvent to produce a polymer-iodide and was subsequently used as a substitution agent in a polar solvent by simply adding the polar solvent, thereby transforming the polymer-iodide to polymer-N3 in one pot. This one-pot synthesis was further applied to obtain N3-chain-end-functionalized polymer brushes on the surface, uniquely controlling the N3 coverage (number density). Using the chain-end N3, the obtained linear and brush polymers were connected to functional molecules via an azide-alkyne click reaction. The attractive features of this system include facile operation, access to unique polymer designs, and no requirement for using excess NaN3. In addition to N3-, thiocyanate (-SCN) and cyanate (-OCN) anions were also studied.
中文翻译:

烷基碘与叠氮化钠的溶剂选择性反应自由基生成和叠氮取代及其在链端官能化聚合物的一锅法合成中的应用
在此,报道了烷基碘 (RI) 与叠氮阴离子 (N3-) 可逆地生成相应烷基 (R•) 的新反应。通过这种新反应,N3- 被用作活性自由基聚合中的有效催化剂,产生了明确定义的聚合物碘化物。一个特别有趣的发现是该反应的溶剂选择性;即,RI 和 N3-在非极性溶剂中生成 R•,而取代产物 R-N3 在极性溶剂中生成。利用这种独特的溶剂选择性,实现了聚合物-N3 的一锅法合成。N3-首先在非极性溶剂中用作活性自由基聚合的催化剂,生成聚合物-碘化物,随后在极性溶剂中通过简单地加入极性溶剂用作取代剂,从而在一锅中将聚合物-碘化物转化为聚合物-N3。进一步应用这种一锅法合成在表面上获得 N3 链端功能化聚合物刷,独特地控制 N3 覆盖率(数密度)。使用链端 N3,获得的线性和刷状聚合物通过叠氮化物-炔烃点击反应连接到功能分子上。该系统的吸引人的特点包括操作简便、可使用独特的聚合物设计,并且不需要使用过量的 NaN3。除了 N3-,还研究了硫氰酸根 (-SCN) 和氰酸根 (-OCN) 阴离子。获得的线性和刷状聚合物通过叠氮化物-炔烃点击反应连接到功能分子上。该系统的吸引人的特点包括操作简便、可使用独特的聚合物设计,并且不需要使用过量的 NaN3。除了 N3-,还研究了硫氰酸根 (-SCN) 和氰酸根 (-OCN) 阴离子。获得的线性和刷状聚合物通过叠氮化物-炔烃点击反应连接到功能分子上。该系统的吸引人的特点包括操作简便、可使用独特的聚合物设计,并且不需要使用过量的 NaN3。除了 N3-,还研究了硫氰酸根 (-SCN) 和氰酸根 (-OCN) 阴离子。
更新日期:2017-07-25
中文翻译:

烷基碘与叠氮化钠的溶剂选择性反应自由基生成和叠氮取代及其在链端官能化聚合物的一锅法合成中的应用
在此,报道了烷基碘 (RI) 与叠氮阴离子 (N3-) 可逆地生成相应烷基 (R•) 的新反应。通过这种新反应,N3- 被用作活性自由基聚合中的有效催化剂,产生了明确定义的聚合物碘化物。一个特别有趣的发现是该反应的溶剂选择性;即,RI 和 N3-在非极性溶剂中生成 R•,而取代产物 R-N3 在极性溶剂中生成。利用这种独特的溶剂选择性,实现了聚合物-N3 的一锅法合成。N3-首先在非极性溶剂中用作活性自由基聚合的催化剂,生成聚合物-碘化物,随后在极性溶剂中通过简单地加入极性溶剂用作取代剂,从而在一锅中将聚合物-碘化物转化为聚合物-N3。进一步应用这种一锅法合成在表面上获得 N3 链端功能化聚合物刷,独特地控制 N3 覆盖率(数密度)。使用链端 N3,获得的线性和刷状聚合物通过叠氮化物-炔烃点击反应连接到功能分子上。该系统的吸引人的特点包括操作简便、可使用独特的聚合物设计,并且不需要使用过量的 NaN3。除了 N3-,还研究了硫氰酸根 (-SCN) 和氰酸根 (-OCN) 阴离子。获得的线性和刷状聚合物通过叠氮化物-炔烃点击反应连接到功能分子上。该系统的吸引人的特点包括操作简便、可使用独特的聚合物设计,并且不需要使用过量的 NaN3。除了 N3-,还研究了硫氰酸根 (-SCN) 和氰酸根 (-OCN) 阴离子。获得的线性和刷状聚合物通过叠氮化物-炔烃点击反应连接到功能分子上。该系统的吸引人的特点包括操作简便、可使用独特的聚合物设计,并且不需要使用过量的 NaN3。除了 N3-,还研究了硫氰酸根 (-SCN) 和氰酸根 (-OCN) 阴离子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号